当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and Reactivity of (μ-Oxo)dimanganese(III,III) and Mononuclear Hydroxomanganese(III) Adducts Supported by Derivatives of an Amide-Containing Pentadentate Ligand
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-12-07 00:00:00 , DOI: 10.1021/acs.inorgchem.8b02794
Derek B. Rice 1 , Aruna Munasinghe 1 , Elizabeth N. Grotemeyer 1 , Andrew D. Burr 1 , Victor W. Day 1 , Timothy A. Jackson 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-12-07 00:00:00 , DOI: 10.1021/acs.inorgchem.8b02794
Derek B. Rice 1 , Aruna Munasinghe 1 , Elizabeth N. Grotemeyer 1 , Andrew D. Burr 1 , Victor W. Day 1 , Timothy A. Jackson 1
Affiliation
|
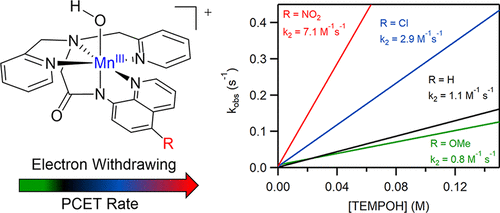
Mononuclear MnIII–hydroxo and dinuclear (μ-oxo)dimanganese(III,III) complexes were prepared using derivatives of the pentadentate, amide-containing dpaq ligand (dpaq = 2-[bis(pyridin-2-ylmethyl)]amino-N-quinolin-8-yl-acetamidate). Each of these ligand derivatives (referred to as dpaq5R) contained a substituent R (where R = OMe, Cl, and NO2) at the 5-position of the quinolinyl group. Generation of the MnIII complexes was achieved by either O2 oxidation of MnII precursors (for [MnII(dpaq5OMe)]+ and [MnII(dpaq5Cl)]+ or PhIO oxidation (for [MnII(dpaq5NO2)]+). For each oxidized complex, 1H NMR experiments provided evidence of a water-dependent equilibrium between paramagnetic [MnIII(OH)(dpaq5R)]+ and an antiferromagnetically coupled [MnIIIMnIII(μ-O)(dpaq5R)2]2+ species in acetonitrile, with the addition of water favoring the MnIII–hydroxo species. This conversion could also be monitored by electronic absorption spectroscopy. Solid-state X-ray crystal structures for each [MnIIIMnIII(μ-O)(dpaq5R)2](OTf)2 complex revealed a nearly linear Mn–O–Mn core (angle of ca. 177°), with short Mn–O distances near 1.79 Å, and a Mn···Mn separation of 3.58 Å. X-ray crystallographic information was also obtained for the mononuclear [MnIII(OH)(dpaq5Cl)](OTf) complex, which has a short Mn–O(H) distance of 1.810(2) Å. The influence of the 5-substituted quinolinyl moiety on the electronic properties of the [MnIII(OH)(dpaq5R)]+ complexes was demonstrated through shifts in a number of 1H NMR resonances, as well as a steady increase in the MnIII/II cyclic voltammetry peak potential in the order [MnIII(OH)(dpaq5OMe)]+ < [MnIII(OH)(dpaq)]+ < [MnIII(OH)(dpaq5Cl)]+ < [MnIII(OH)(dpaq5NO2)]+. These changes in oxidizing power of the MnIII–hydroxo adducts translated to only modest rate enhancements for TEMPOH oxidation by the [MnIII(OH)(dpaq5R)]+ complexes, with the most reactive [MnIII(OH)(dpaq5NO2)]+ complex showing a second-order rate constant only 9-fold larger than that of the least reactive [MnIII(OH)(dpaq5OMe)]+ complex. These modest rate changes were understood on the basis of density functional theory (DFT)-computed pKa values for the corresponding [MnII(OH2)(dpaq5R)]+ complexes. Collectively, the experimental and DFT results reveal that the 5-substituted quinolinyl groups have an inverse influence on electron and proton affinity for the MnIII–hydroxo unit.
中文翻译:

含酰胺的五齿配体衍生物支持的(μ-氧代)二锰(III,III)和单核羟基锰(III)加合物的结构和反应性
单核锰III -hydroxo和双核(μ-氧代)二锰(III,III)使用五齿,含酰胺dpaq配体(dpaq = 2- [双(吡啶-2-基甲基)]氨基的衍生物制备络合物Ñ -喹啉-8-乙酰氨基乙酸)。这些配体衍生物(称为dpaq 5R)各自在喹啉基的5位上具有取代基R(R = OMe,Cl和NO 2)。Mn III复合物的生成是通过Mn II前体的O 2氧化(对于[Mn II(dpaq 5OMe)] +和[Mn II(dpaq 5Cl)] +或PhIO氧化(对于[Mn II(dpaq 5NO 2)] +)。对于每种氧化的络合物,1 H NMR实验提供了顺磁性[Mn III(OH)(dpaq 5R)] +和反铁磁耦合[Mn III Mn III(μ-O)(dpaq 5R)2 ] 2+物种在乙腈中,通过加入水有利于使Mn III -hydroxo物种。该转化也可以通过电子吸收光谱法监测。每个[Mn III Mn的固态X射线晶体结构III(μ-O)(dpaq 5R)2 ](OTf)2络合物显示出接近线性的Mn-O-Mn核(角度约为177°),Mn-O距离短,接近1.79Å,并且Mn· ··锰分离度为3.58Å。还获得了单核[Mn III(OH)(dpaq 5Cl)](OTf)配合物的X射线晶体学信息,该配合物的Mn-O(H)短距离为1.810(2)Å。通过多个1 H NMR共振的位移以及Mn的稳定增加,证明了5-取代的喹啉基部分对[Mn III(OH)(dpaq 5R)] +配合物的电子性能的影响。III / II[Mn III(OH)(dpaq 5OMe)] + <[Mn III(OH)(dpaq)] + <[Mn III(OH)(dpaq 5Cl)] + <[Mn III(OH) )(dpaq 5NO 2)] +。Mn III –羟基加合物的氧化能力的这些变化仅转化为[Mn III(OH)(dpaq 5R)] +配合物对TEMPOH的适度提高,其中反应性最高的[Mn III(OH)(dpaq 5NO)2)] +络合物的二级速率常数仅比反应性最低的[Mn III(OH)(dpaq 5OMe)] +络合物的常数速率常数大9倍。基于密度泛函理论(DFT)计算的相应[Mn II(OH 2)(dpaq 5R)] +配合物的p K a值,可以理解这些适度的速率变化。总的来说,实验和DFT结果表明,5-取代的喹啉基对Mn III –羟基单元的电子和质子亲和力具有相反的影响。
更新日期:2018-12-07
中文翻译:

含酰胺的五齿配体衍生物支持的(μ-氧代)二锰(III,III)和单核羟基锰(III)加合物的结构和反应性
单核锰III -hydroxo和双核(μ-氧代)二锰(III,III)使用五齿,含酰胺dpaq配体(dpaq = 2- [双(吡啶-2-基甲基)]氨基的衍生物制备络合物Ñ -喹啉-8-乙酰氨基乙酸)。这些配体衍生物(称为dpaq 5R)各自在喹啉基的5位上具有取代基R(R = OMe,Cl和NO 2)。Mn III复合物的生成是通过Mn II前体的O 2氧化(对于[Mn II(dpaq 5OMe)] +和[Mn II(dpaq 5Cl)] +或PhIO氧化(对于[Mn II(dpaq 5NO 2)] +)。对于每种氧化的络合物,1 H NMR实验提供了顺磁性[Mn III(OH)(dpaq 5R)] +和反铁磁耦合[Mn III Mn III(μ-O)(dpaq 5R)2 ] 2+物种在乙腈中,通过加入水有利于使Mn III -hydroxo物种。该转化也可以通过电子吸收光谱法监测。每个[Mn III Mn的固态X射线晶体结构III(μ-O)(dpaq 5R)2 ](OTf)2络合物显示出接近线性的Mn-O-Mn核(角度约为177°),Mn-O距离短,接近1.79Å,并且Mn· ··锰分离度为3.58Å。还获得了单核[Mn III(OH)(dpaq 5Cl)](OTf)配合物的X射线晶体学信息,该配合物的Mn-O(H)短距离为1.810(2)Å。通过多个1 H NMR共振的位移以及Mn的稳定增加,证明了5-取代的喹啉基部分对[Mn III(OH)(dpaq 5R)] +配合物的电子性能的影响。III / II[Mn III(OH)(dpaq 5OMe)] + <[Mn III(OH)(dpaq)] + <[Mn III(OH)(dpaq 5Cl)] + <[Mn III(OH) )(dpaq 5NO 2)] +。Mn III –羟基加合物的氧化能力的这些变化仅转化为[Mn III(OH)(dpaq 5R)] +配合物对TEMPOH的适度提高,其中反应性最高的[Mn III(OH)(dpaq 5NO)2)] +络合物的二级速率常数仅比反应性最低的[Mn III(OH)(dpaq 5OMe)] +络合物的常数速率常数大9倍。基于密度泛函理论(DFT)计算的相应[Mn II(OH 2)(dpaq 5R)] +配合物的p K a值,可以理解这些适度的速率变化。总的来说,实验和DFT结果表明,5-取代的喹啉基对Mn III –羟基单元的电子和质子亲和力具有相反的影响。































 京公网安备 11010802027423号
京公网安备 11010802027423号