当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Manipulation of prenylation reactions by structure-based engineering of bacterial indolactam prenyltransferases.
Nature Communications ( IF 14.7 ) Pub Date : 2016-Mar-08 , DOI: 10.1038/ncomms10849 Takahiro Mori , Lihan Zhang , Takayoshi Awakawa , Shotaro Hoshino , Masahiro Okada , Hiroyuki Morita , Ikuro Abe
Nature Communications ( IF 14.7 ) Pub Date : 2016-Mar-08 , DOI: 10.1038/ncomms10849 Takahiro Mori , Lihan Zhang , Takayoshi Awakawa , Shotaro Hoshino , Masahiro Okada , Hiroyuki Morita , Ikuro Abe
|
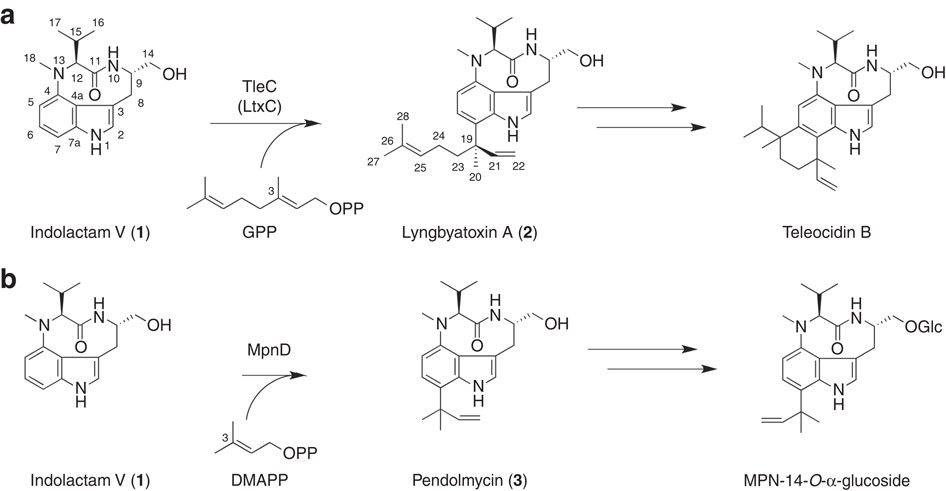
Prenylation reactions play crucial roles in controlling the activities of biomolecules. Bacterial prenyltransferases, TleC from Streptomyces blastmyceticus and MpnD from Marinactinospora thermotolerans, catalyse the 'reverse' prenylation of (-)-indolactam V at the C-7 position of the indole ring with geranyl pyrophosphate or dimethylallyl pyrophosphate, to produce lyngbyatoxin or pendolmycin, respectively. Using in vitro analyses, here we show that both TleC and MpnD exhibit relaxed substrate specificities and accept various chain lengths (C5-C25) of the prenyl donors. Comparisons of the crystal structures and their ternary complexes with (-)-indolactam V and dimethylallyl S-thiophosphate revealed the intimate structural details of the enzyme-catalysed 'reverse' prenylation reactions and identified the active-site residues governing the selection of the substrates. Furthermore, structure-based enzyme engineering successfully altered the preference for the prenyl chain length of the substrates, as well as the regio- and stereo-selectivities of the prenylation reactions, to produce a series of unnatural novel indolactams.
中文翻译:

通过基于结构的细菌吲哚内酰胺异戊二烯基转移酶工程来控制异戊烯化反应。
烯丙基化反应在控制生物分子的活性中起关键作用。细菌异戊二烯基转移酶,来自稻瘟菌的链霉菌的TleC和来自耐热链霉菌的MpnD,分别催化香叶基焦磷酸酯或焦磷酸二甲基烯丙酯在吲哚环C-7位上的(-)-吲哚内酰胺V的“反向”异戊烯酸V,以产生聚霉菌素。 。使用体外分析,在这里我们显示TleC和MpnD均显示松弛的底物特异性,并接受异戊二烯基供体的各种链长(C5-C25)。与(-)-吲哚内酰胺V和二甲基烯丙基S-硫代磷酸酯的晶体结构及其三元络合物的比较揭示了酶催化的“反向”的紧密结构细节。异戊二烯基化反应,并确定了控制底物选择的活性位点残基。此外,基于结构的酶工程成功地改变了对底物异戊二烯链长度的偏好,以及异戊二烯化反应的区域选择性和立体选择性,从而产生了一系列非天然的新型吲哚内酰胺。
更新日期:2016-03-11
中文翻译:

通过基于结构的细菌吲哚内酰胺异戊二烯基转移酶工程来控制异戊烯化反应。
烯丙基化反应在控制生物分子的活性中起关键作用。细菌异戊二烯基转移酶,来自稻瘟菌的链霉菌的TleC和来自耐热链霉菌的MpnD,分别催化香叶基焦磷酸酯或焦磷酸二甲基烯丙酯在吲哚环C-7位上的(-)-吲哚内酰胺V的“反向”异戊烯酸V,以产生聚霉菌素。 。使用体外分析,在这里我们显示TleC和MpnD均显示松弛的底物特异性,并接受异戊二烯基供体的各种链长(C5-C25)。与(-)-吲哚内酰胺V和二甲基烯丙基S-硫代磷酸酯的晶体结构及其三元络合物的比较揭示了酶催化的“反向”的紧密结构细节。异戊二烯基化反应,并确定了控制底物选择的活性位点残基。此外,基于结构的酶工程成功地改变了对底物异戊二烯链长度的偏好,以及异戊二烯化反应的区域选择性和立体选择性,从而产生了一系列非天然的新型吲哚内酰胺。






























 京公网安备 11010802027423号
京公网安备 11010802027423号