当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Air-Exposure Degradation Chemistry at a Nanoscale of Layered Oxide Cathodes for Sodium-Ion Batteries
Nano Letters ( IF 9.6 ) Pub Date : 2018-12-05 00:00:00 , DOI: 10.1021/acs.nanolett.8b03637 Ya You 1 , Andrei Dolocan 1 , Wangda Li 1 , Arumugam Manthiram 1
Nano Letters ( IF 9.6 ) Pub Date : 2018-12-05 00:00:00 , DOI: 10.1021/acs.nanolett.8b03637 Ya You 1 , Andrei Dolocan 1 , Wangda Li 1 , Arumugam Manthiram 1
Affiliation
|
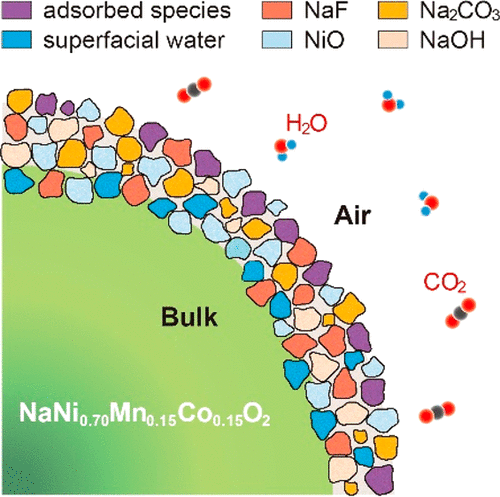
Undesired reactions between layered sodium transition-metal oxide cathodes and air impede their utilization in practical sodium-ion batteries. Consequently, a fundamental understanding of how layered oxide cathodes degrade in air is of paramount importance, but it has not been fully understood yet. Here a comprehensive study on a model material NaNi0.7Mn0.15Co0.15O2 reveals its reaction chemistry with air and the dynamic evolution of the degradation species upon air exposure. We find that besides the extraction of Na+ ions from the crystal lattice to form NaOH, Na2CO3, and Na2CO3·H2O in contact with air, nickel ions gradually dissolve from the bulk to form NiO and accumulate on the particle surface as revealed by subnanometer surface-sensitive time-of-flight secondary ion mass spectroscopy. The degradation species on the surface are insulating, leading to an increase in interfacial resistance and declined electrochemical performance. We also demonstrate a feasible surface coating strategy for suppressing the unfavorable degradation process. Understanding the degradation mechanism at a nanoscale can facilitate the future development of high-energy cathodes for sodium-ion batteries.
中文翻译:

了解用于钠离子电池的纳米级分层氧化物阴极的空气降解化学
层状钠过渡金属氧化物阴极与空气之间的不良反应阻碍了它们在实际的钠离子电池中的利用。因此,最基本的了解层状氧化阴极在空气中的降解方式至关重要,但尚未完全了解。在这里,对模型材料NaNi 0.7 Mn 0.15 Co 0.15 O 2的全面研究揭示了其与空气的化学反应以及暴露于空气后降解物质的动态演变。我们发现,除了从晶格中提取Na +离子以形成NaOH外,Na 2 CO 3和Na 2 CO 3 ·H 2O与空气接触后,镍离子逐渐从主体中溶解而形成NiO,并在颗粒表面积聚,这是通过亚纳米级表面敏感的飞行时间二次离子质谱分析得出的。表面上的降解物质是绝缘的,导致界面电阻增加和电化学性能下降。我们还证明了抑制不利的降解过程的可行的表面涂层策略。了解纳米级的降解机理可以促进钠离子电池高能阴极的未来发展。
更新日期:2018-12-05
中文翻译:

了解用于钠离子电池的纳米级分层氧化物阴极的空气降解化学
层状钠过渡金属氧化物阴极与空气之间的不良反应阻碍了它们在实际的钠离子电池中的利用。因此,最基本的了解层状氧化阴极在空气中的降解方式至关重要,但尚未完全了解。在这里,对模型材料NaNi 0.7 Mn 0.15 Co 0.15 O 2的全面研究揭示了其与空气的化学反应以及暴露于空气后降解物质的动态演变。我们发现,除了从晶格中提取Na +离子以形成NaOH外,Na 2 CO 3和Na 2 CO 3 ·H 2O与空气接触后,镍离子逐渐从主体中溶解而形成NiO,并在颗粒表面积聚,这是通过亚纳米级表面敏感的飞行时间二次离子质谱分析得出的。表面上的降解物质是绝缘的,导致界面电阻增加和电化学性能下降。我们还证明了抑制不利的降解过程的可行的表面涂层策略。了解纳米级的降解机理可以促进钠离子电池高能阴极的未来发展。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号