当前位置:
X-MOL 学术
›
JAMA Oncol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Assessment of ERBB2/HER2 Status in HER2-Equivocal Breast Cancers by FISH and 2013/2014 ASCO-CAP Guidelines.
JAMA Oncology ( IF 22.5 ) Pub Date : 2019-03-01 , DOI: 10.1001/jamaoncol.2018.6012 Michael F Press 1 , Jose A Seoane 2 , Christina Curtis 2 , Emmanuel Quinaux 3 , Roberta Guzman 1 , Guido Sauter 4 , Wolfgang Eiermann 5 , John R Mackey 6 , Nicholas Robert 7 , Tadeusz Pienkowski 8 , John Crown 9 , Miguel Martin 10 , Vicente Valero 11 , Valerie Bee 12 , Yanling Ma 1 , Ivonne Villalobos 1 , Dennis J Slamon 13
JAMA Oncology ( IF 22.5 ) Pub Date : 2019-03-01 , DOI: 10.1001/jamaoncol.2018.6012 Michael F Press 1 , Jose A Seoane 2 , Christina Curtis 2 , Emmanuel Quinaux 3 , Roberta Guzman 1 , Guido Sauter 4 , Wolfgang Eiermann 5 , John R Mackey 6 , Nicholas Robert 7 , Tadeusz Pienkowski 8 , John Crown 9 , Miguel Martin 10 , Vicente Valero 11 , Valerie Bee 12 , Yanling Ma 1 , Ivonne Villalobos 1 , Dennis J Slamon 13
Affiliation
|
Importance
The 2013/2014 American Society of Clinical Oncology and College of American Pathologists (ASCO-CAP) guidelines for HER2 testing by fluorescence in situ hybridization (FISH) designated an "equivocal" category (average HER2 copies per tumor cell ≥4-6 with HER2/CEP17 ratio <2.0) to be resolved as negative or positive by assessments with alternative control probes. Approximately 4% to 12% of all invasive breast cancers are characterized as HER2-equivocal based on FISH.
Objective
To evaluate the following hypotheses: (1) genetic loci used as alternative controls are heterozygously deleted in a substantial proportion of breast cancers; (2) use of these loci for assessment of HER2 by FISH leads to false-positive assessments; and (3) these HER2 false-positive breast cancer patients have outcomes that do not differ from clinical outcomes for patients with HER2-negative breast cancer.
Design, Setting, and Participants
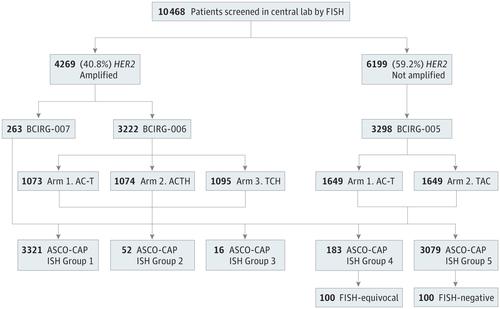
We retrospectively assessed the use of chromosome 17 p-arm and q-arm alternative control genomic sites (TP53, D17S122, SMS, RARA, TOP2A), as recommended by the 2013/2014 ASCO-CAP guidelines for HER2 testing, in patients whose data were available through Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) and whose tissues were available through the Breast Cancer International Research Group clinical trials. We used data from an international cohort database of invasive breast cancers (1980 participants) and international clinical trial of adjuvant chemotherapy in invasive, node-positive breast cancer patients.
Main Outcomes and Measures
The primary objectives were to (1) assess frequency of heterozygous deletions in chromosome 17 genomic sites used as FISH internal controls for evaluation of HER2 status among HER2-equivocal cancers; (2) characterize impact of using deleted sites for determination of HER2-to-internal-control-gene ratios; (3) assess HER2 protein expression in each subgroup; and (4) compare clinical outcomes for each subgroup.
Results
Of the 1980 patients in METABRIC,1915 patients were fully evaluated. In addition, 100 HER2-equivocal breast cancers by FISH and 100 comparator FISH-negative breast cancers from the BCIRG-005 trial were analyzed. Heterozygous deletions, particularly in specific p-arm sites, were common in both HER2-amplified and HER2-not-amplified breast cancers. Use of alternative control probes from these regions to assess HER2 by FISH in HER2-equivocal as well as HER2-not-amplified breast cancers resulted in high rates of false-positive ratios (HER2-to-alternative control ratio ≥2.0) owing to heterozygous deletions of control p-arm genomic sites used in ratio denominators. Misclassification of HER2 status was observed not only in breast cancers with ASCO-CAP equivocal status but also in breast cancers with an average of fewer than 4.0 HER2 copies per tumor cell when using alternative control probes.
Conclusions and Relevance
The indiscriminate use of alternative control probes to calculate HER2 FISH ratios in HER2-equivocal breast cancers may lead to false-positive interpretations of HER2 status resulting from unrecognized heterozygous deletions in 1 or more of these alternative control genomic sites and incorrect HER2 ratio determinations.
中文翻译:

通过FISH和2013/2014 ASCO-CAP指南评估HER2等同型乳腺癌中ERBB2 / HER2的状态。
重要性2013/2014美国临床肿瘤学会和美国病理学家学院(ASCO-CAP)通过荧光原位杂交(FISH)进行HER2检测的指南指定了“明确的”类别(每个≥4-6的肿瘤细胞平均HER2拷贝为HER2 / CEP17比率<2.0)可通过使用其他对照探针的评估解决为阴性或阳性。基于FISH,所有浸润性乳腺癌中约有4%至12%被定性为HER2。目的评估以下假设:(1)在相当大比例的乳腺癌中,杂合性缺失了作为替代对照的基因位点;(2)使用这些基因座通过FISH评估HER2会导致假阳性评估;(3)这些HER2假阳性乳腺癌患者的结局与HER2阴性乳腺癌患者的临床结局没有差异。设计,设置和参与者我们按照2013/2014 ASCO-CAP HER2指南的建议,回顾性评估了17号染色体p臂和q臂替代控制基因组位点(TP53,D17S122,SMS,RARA,TOP2A)的使用测试可通过乳腺癌国际联合会分子分类法(METABRIC)获得数据,且组织可通过乳腺癌国际研究组临床试验获得。我们使用了国际浸润性乳腺癌队列数据库(1980年参加者)中的数据以及浸润性淋巴结阳性乳腺癌患者辅助化疗的国际临床试验。主要结果和措施主要目标是(1)评估17染色体基因组位点中杂合性缺失的频率,这些位点用作FISH内部对照,以评估HER2特异性癌症中HER2的状态;(2)表征使用删除的位点来确定HER2与内部控制基因之比的影响;(3)评估每个亚组中的HER2蛋白表达;(4)比较每个亚组的临床结局。结果在METABRIC的1980例患者中,对1915例患者进行了全面评估。此外,还分析了来自BCIRG-005试验的100例FISH HER2明确乳腺癌和100例FISH阴性对照乳腺癌。杂合子缺失,特别是在特定的p-臂位点,在HER2扩增和HER2非扩增的乳腺癌中都很常见。使用这些区域的替代对照探针通过FISH评估HER2特异性和HER2非扩增型乳腺癌中的HER2,由于杂合,导致假阳性率较高(HER2与其他对照率≥2.0)。比例分母中使用的控制p臂基因组位点的缺失。当使用替代对照探针时,不仅在具有ASCO-CAP模棱两可状态的乳腺癌中,而且在每个肿瘤细胞平均少于4.0 HER2拷贝的乳腺癌中,都观察到HER2状态的分类错误。
更新日期:2019-03-15
中文翻译:

通过FISH和2013/2014 ASCO-CAP指南评估HER2等同型乳腺癌中ERBB2 / HER2的状态。
重要性2013/2014美国临床肿瘤学会和美国病理学家学院(ASCO-CAP)通过荧光原位杂交(FISH)进行HER2检测的指南指定了“明确的”类别(每个≥4-6的肿瘤细胞平均HER2拷贝为HER2 / CEP17比率<2.0)可通过使用其他对照探针的评估解决为阴性或阳性。基于FISH,所有浸润性乳腺癌中约有4%至12%被定性为HER2。目的评估以下假设:(1)在相当大比例的乳腺癌中,杂合性缺失了作为替代对照的基因位点;(2)使用这些基因座通过FISH评估HER2会导致假阳性评估;(3)这些HER2假阳性乳腺癌患者的结局与HER2阴性乳腺癌患者的临床结局没有差异。设计,设置和参与者我们按照2013/2014 ASCO-CAP HER2指南的建议,回顾性评估了17号染色体p臂和q臂替代控制基因组位点(TP53,D17S122,SMS,RARA,TOP2A)的使用测试可通过乳腺癌国际联合会分子分类法(METABRIC)获得数据,且组织可通过乳腺癌国际研究组临床试验获得。我们使用了国际浸润性乳腺癌队列数据库(1980年参加者)中的数据以及浸润性淋巴结阳性乳腺癌患者辅助化疗的国际临床试验。主要结果和措施主要目标是(1)评估17染色体基因组位点中杂合性缺失的频率,这些位点用作FISH内部对照,以评估HER2特异性癌症中HER2的状态;(2)表征使用删除的位点来确定HER2与内部控制基因之比的影响;(3)评估每个亚组中的HER2蛋白表达;(4)比较每个亚组的临床结局。结果在METABRIC的1980例患者中,对1915例患者进行了全面评估。此外,还分析了来自BCIRG-005试验的100例FISH HER2明确乳腺癌和100例FISH阴性对照乳腺癌。杂合子缺失,特别是在特定的p-臂位点,在HER2扩增和HER2非扩增的乳腺癌中都很常见。使用这些区域的替代对照探针通过FISH评估HER2特异性和HER2非扩增型乳腺癌中的HER2,由于杂合,导致假阳性率较高(HER2与其他对照率≥2.0)。比例分母中使用的控制p臂基因组位点的缺失。当使用替代对照探针时,不仅在具有ASCO-CAP模棱两可状态的乳腺癌中,而且在每个肿瘤细胞平均少于4.0 HER2拷贝的乳腺癌中,都观察到HER2状态的分类错误。

































 京公网安备 11010802027423号
京公网安备 11010802027423号