当前位置:
X-MOL 学术
›
Gene Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Immunosuppression overcomes insulin- and vector-specific immune responses that limit efficacy of AAV2/8-mediated insulin gene therapy in NOD mice.
Gene Therapy ( IF 4.6 ) Pub Date : 2018-12-04 , DOI: 10.1038/s41434-018-0052-5 Asha Recino 1 , Shu Uin Gan 2 , Kian Chuan Sia 2 , Yvonne Sawyer 1 , Jenny Trendell 1 , Richard Kay 3 , Fiona M Gribble 3 , Frank Reimann 3 , Rob Foale 4 , Maria Notaridou 5 , Nick Holmes 1 , Andrew Lever 6, 7 , Kok Onn Lee 7 , Amit Nathwani 5 , Anne Cooke 1 , Roy Calne 2, 7, 8 , Maja Wallberg 1
Gene Therapy ( IF 4.6 ) Pub Date : 2018-12-04 , DOI: 10.1038/s41434-018-0052-5 Asha Recino 1 , Shu Uin Gan 2 , Kian Chuan Sia 2 , Yvonne Sawyer 1 , Jenny Trendell 1 , Richard Kay 3 , Fiona M Gribble 3 , Frank Reimann 3 , Rob Foale 4 , Maria Notaridou 5 , Nick Holmes 1 , Andrew Lever 6, 7 , Kok Onn Lee 7 , Amit Nathwani 5 , Anne Cooke 1 , Roy Calne 2, 7, 8 , Maja Wallberg 1
Affiliation
|
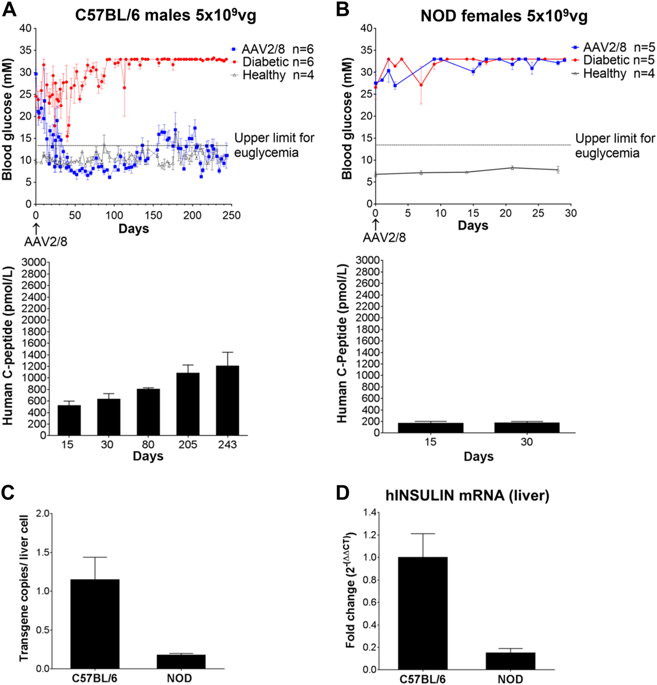
We report the restoration of euglycaemia in chemically induced diabetic C57BL/6 mice and spontaneously diabetic Non Obese Diabetic (NOD) mice by intravenous systemic administration of a single-stranded adeno-associated virus (ssAAV2/8) codon optimised (co) vector encoding furin cleavable human proinsulin under a liver-specific promoter. There were no immunological barriers to efficacy of insulin gene therapy in chemically induced C57BL/6 mice, which enjoyed long-lasting correction of hyperglycaemia after therapy, up to 250 days. Euglycaemia was also restored in spontaneously diabetic NOD mice, although these mice required a 7-10-fold higher dose of vector to achieve similar efficacy as the C57BL/6 mice and the immunodeficient NODscid mice. We detected CD8+ T cell reactivity to insulin and mild inflammatory infiltration in the livers of gene therapy recipient NOD mice, neither of which were observed in the treated C57BL/6 mice. Efficacy of the gene therapy in NOD mice was partially improved by targeting the immune system with anti-CD4 antibody treatment, while transfer of NOD mouse AAV2/8-reactive serum to recipients prevented successful restoration of euglycaemia in AAV2/8-HLP-hINSco-treated NODscid mice. Our data indicate that both immune cells and antibodies form a barrier to successful restoration of euglycaemia in autoimmune diabetic recipient mice with insulin gene therapy, but that this barrier can be overcome by increasing the dose of vector and by suppressing immune responses.
中文翻译:

免疫抑制克服了胰岛素和载体特异性免疫反应,这些免疫反应限制了 AAV2/8 介导的胰岛素基因治疗在 NOD 小鼠中的疗效。
我们报道了通过静脉内全身注射编码弗林蛋白酶的单链腺相关病毒 (ssAAV2/8) 密码子优化 (co) 载体,化学诱导的糖尿病 C57BL/6 小鼠和自发性糖尿病非肥胖糖尿病 (NOD) 小鼠的血糖恢复正常在肝脏特异性启动子的作用下可裂解的人胰岛素原。在化学诱导的 C57BL/6 小鼠中,胰岛素基因治疗的功效不存在免疫障碍,治疗后高血糖得到持久纠正,长达 250 天。自发性糖尿病 NOD 小鼠的血糖也得到恢复,尽管这些小鼠需要 7-10 倍高剂量的载体才能达到与 C57BL/6 小鼠和免疫缺陷 NODscid 小鼠相似的功效。我们在基因治疗受体 NOD 小鼠的肝脏中检测到 CD8+ T 细胞对胰岛素的反应性和轻度炎症浸润,而在治疗的 C57BL/6 小鼠中未观察到这两种情况。通过使用抗 CD4 抗体治疗靶向免疫系统,NOD 小鼠的基因治疗效果部分得到改善,而将 NOD 小鼠 AAV2/8 反应性血清转移至受体体内,则阻止了 AAV2/8-HLP-hINSco 中血糖正常的成功恢复。治疗 NODscid 小鼠。我们的数据表明,免疫细胞和抗体都形成了胰岛素基因治疗的自身免疫糖尿病受体小鼠成功恢复血糖正常的障碍,但可以通过增加载体剂量和抑制免疫反应来克服这一障碍。
更新日期:2019-05-16
中文翻译:

免疫抑制克服了胰岛素和载体特异性免疫反应,这些免疫反应限制了 AAV2/8 介导的胰岛素基因治疗在 NOD 小鼠中的疗效。
我们报道了通过静脉内全身注射编码弗林蛋白酶的单链腺相关病毒 (ssAAV2/8) 密码子优化 (co) 载体,化学诱导的糖尿病 C57BL/6 小鼠和自发性糖尿病非肥胖糖尿病 (NOD) 小鼠的血糖恢复正常在肝脏特异性启动子的作用下可裂解的人胰岛素原。在化学诱导的 C57BL/6 小鼠中,胰岛素基因治疗的功效不存在免疫障碍,治疗后高血糖得到持久纠正,长达 250 天。自发性糖尿病 NOD 小鼠的血糖也得到恢复,尽管这些小鼠需要 7-10 倍高剂量的载体才能达到与 C57BL/6 小鼠和免疫缺陷 NODscid 小鼠相似的功效。我们在基因治疗受体 NOD 小鼠的肝脏中检测到 CD8+ T 细胞对胰岛素的反应性和轻度炎症浸润,而在治疗的 C57BL/6 小鼠中未观察到这两种情况。通过使用抗 CD4 抗体治疗靶向免疫系统,NOD 小鼠的基因治疗效果部分得到改善,而将 NOD 小鼠 AAV2/8 反应性血清转移至受体体内,则阻止了 AAV2/8-HLP-hINSco 中血糖正常的成功恢复。治疗 NODscid 小鼠。我们的数据表明,免疫细胞和抗体都形成了胰岛素基因治疗的自身免疫糖尿病受体小鼠成功恢复血糖正常的障碍,但可以通过增加载体剂量和抑制免疫反应来克服这一障碍。































 京公网安备 11010802027423号
京公网安备 11010802027423号