当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Straightforward and Regioselective C–H Alkenylation of Indoles with Alkenyl Bromides: Scope and Mechanistic Aspect
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-12-04 00:00:00 , DOI: 10.1021/acscatal.8b04267
Rahul A. Jagtap , C. P. Vinod , Benudhar Punji
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-12-04 00:00:00 , DOI: 10.1021/acscatal.8b04267
Rahul A. Jagtap , C. P. Vinod , Benudhar Punji
|
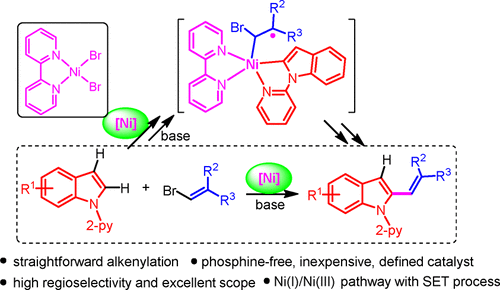
Nickel-catalyzed regioselective C–H bond alkenylation of indoles and related heteroarenes with alkenyl bromides is accomplished under relatively mild conditions. This method allows the straightforward synthesis of C-2 alkenylated indoles employing an air-stable and well-defined nickel catalyst, (bpy)NiBr2, providing a solution to the limitations associated with hydroindolation and oxidative alkenylation. The reaction conceded the coupling of indole derivatives with various alkenyl bromides, such as aromatic and heteroaromatics, α- and β-substituted as well as exo- and endo-cyclic alkenyl compounds. An extensive mechanistic investigation, including controlled study, reactivity experiments, kinetics and labeling studies, and EPR and XPS analyses, highlights that the alkenylation proceeds through a single-electron transfer process comprising an odd-electron oxidative addition of alkenyl bromide. Furthermore, the alkenylation operates via a probable Ni(I)/Ni(III) pathway involving the rate-limiting C–H nickelation of indole.
中文翻译:

镍催化的吲哚与烯丙基溴的直通式和区域选择性CH H烯基化:范围和机理
吲哚和相关杂芳烃与烯基溴的镍催化区域选择性C–H键烯基化反应是在相对温和的条件下完成的。该方法允许使用空气稳定且定义明确的镍催化剂(bpy)NiBr 2来直接合成C-2烯基化的吲哚,从而解决了与氢化吲哚化和氧化烯基化有关的局限性。该反应使吲哚衍生物与各种烯基溴化物(如芳族和杂芳族化合物,α-和β-取代的以及exo-和endo)偶联。-环烯基化合物。广泛的机械研究,包括对照研究,反应性实验,动力学和标记研究,以及EPR和XPS分析,突出表明烯基化过程是通过单电子转移过程进行的,该过程包括烯基溴的奇数电子氧化加成。此外,烯基化反应是通过可能的Ni(I)/ Ni(III)途径进行的,该途径涉及吲哚的限速C-H镍化反应。
更新日期:2018-12-04
中文翻译:

镍催化的吲哚与烯丙基溴的直通式和区域选择性CH H烯基化:范围和机理
吲哚和相关杂芳烃与烯基溴的镍催化区域选择性C–H键烯基化反应是在相对温和的条件下完成的。该方法允许使用空气稳定且定义明确的镍催化剂(bpy)NiBr 2来直接合成C-2烯基化的吲哚,从而解决了与氢化吲哚化和氧化烯基化有关的局限性。该反应使吲哚衍生物与各种烯基溴化物(如芳族和杂芳族化合物,α-和β-取代的以及exo-和endo)偶联。-环烯基化合物。广泛的机械研究,包括对照研究,反应性实验,动力学和标记研究,以及EPR和XPS分析,突出表明烯基化过程是通过单电子转移过程进行的,该过程包括烯基溴的奇数电子氧化加成。此外,烯基化反应是通过可能的Ni(I)/ Ni(III)途径进行的,该途径涉及吲哚的限速C-H镍化反应。

































 京公网安备 11010802027423号
京公网安备 11010802027423号