Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigating the Selectivity of Allosteric Inhibitors for Mutant T790M EGFR over Wild Type Using Molecular Dynamics and Binding Free Energy Calculations
ACS Omega ( IF 3.7 ) Pub Date : 2018-12-04 00:00:00 , DOI: 10.1021/acsomega.8b03256 Annachiara Tinivella 1 , Giulio Rastelli 1
ACS Omega ( IF 3.7 ) Pub Date : 2018-12-04 00:00:00 , DOI: 10.1021/acsomega.8b03256 Annachiara Tinivella 1 , Giulio Rastelli 1
Affiliation
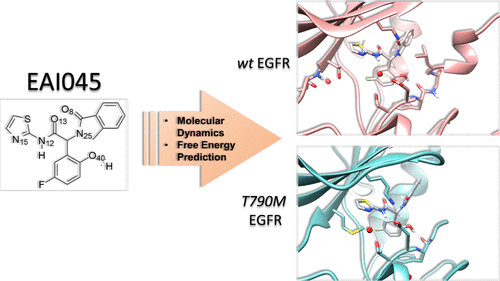
|
The recent discovery of the fourth generation EAI045 allosteric inhibitor, which potently and selectively inhibits mutant EGFR, represents an important step forward for the treatment of non-small cell lung cancer. However, the structural determinants of EAI045 selectivity with respect to the wild type (wt) protein have not been fully investigated. To this aim, we performed a comparative analysis of long-scale molecular dynamics simulations and binding free energy calculations on wt and T790M EGFR in complexes with the EAI001 and EAI045 allosteric ligands. Unexpectedly, we found that the observed selectivity for T790M EGFR over wt is not due to more favorable interactions of the two ligands with the mutated gatekeeper residue, as previously suggested. Rather, the allosteric ligands were engaged in a direct hydrogen bond with the Asp855 residue of the DFG motif in mutant T790M but not in wt, in which the hydrogen bond was found to be water-mediated. Per-residue decomposition of binding free energies suggests that the loss of a direct interaction with Asp855 is the main cause of inhibitor selectivity. Moreover, the possibility that the allosteric ligands and adenosine triphosphate may have synergistic binding effects, as previously observed in MEK allosteric inhibitors, was investigated. Altogether, the results suggest that ligand selectivity arises from direct hydrogen bonds with the Asp855 side chain, and that the design of mutant-selective inhibitors should be focused on ligands that form direct hydrogen bonds with Asp855 in T790M EGFR but not in wt EGFR. These results may provide useful hints for future structural design of mutant-selective allosteric inhibitors that spare wt EGFR, which is a highly desirable goal.
中文翻译:

使用分子动力学和结合自由能计算研究变构抑制剂对野生型突变型T790M EGFR的选择性
第四代EAI045变构抑制剂的最新发现可有效且选择性地抑制突变型EGFR,代表了治疗非小细胞肺癌的重要一步。但是,尚未针对EAI045选择性针对野生型(wt)蛋白的结构决定因素。为此,我们对具有EAI001和EAI045变构配体的复合物中wt和T790M EGFR进行了长期分子动力学模拟和结合自由能计算的比较分析。出乎意料的是,我们发现观察到的针对T790M EGFR的wt选择性并非是由于两个配体与突变的守门员残基之间更有利的相互作用,如先前所建议的那样。相当,变构配体与突变体T790M中DFG基序的Asp855残基直接氢键结合,但不与wt结合,其中氢键被发现是水介导的。结合自由能的每个残基分解表明与Asp855的直接相互作用的丧失是抑制剂选择性的主要原因。而且,如先前在MEK变构抑制剂中观察到的,研究了变构配体和三磷酸腺苷可能具有协同结合作用的可能性。总之,结果表明配体选择性源自与Asp855侧链的直接氢键,而突变选择性抑制剂的设计应侧重于在T790M EGFR中而非与wt EGFR中与Asp855形成直接氢键的配体。
更新日期:2018-12-04
中文翻译:

使用分子动力学和结合自由能计算研究变构抑制剂对野生型突变型T790M EGFR的选择性
第四代EAI045变构抑制剂的最新发现可有效且选择性地抑制突变型EGFR,代表了治疗非小细胞肺癌的重要一步。但是,尚未针对EAI045选择性针对野生型(wt)蛋白的结构决定因素。为此,我们对具有EAI001和EAI045变构配体的复合物中wt和T790M EGFR进行了长期分子动力学模拟和结合自由能计算的比较分析。出乎意料的是,我们发现观察到的针对T790M EGFR的wt选择性并非是由于两个配体与突变的守门员残基之间更有利的相互作用,如先前所建议的那样。相当,变构配体与突变体T790M中DFG基序的Asp855残基直接氢键结合,但不与wt结合,其中氢键被发现是水介导的。结合自由能的每个残基分解表明与Asp855的直接相互作用的丧失是抑制剂选择性的主要原因。而且,如先前在MEK变构抑制剂中观察到的,研究了变构配体和三磷酸腺苷可能具有协同结合作用的可能性。总之,结果表明配体选择性源自与Asp855侧链的直接氢键,而突变选择性抑制剂的设计应侧重于在T790M EGFR中而非与wt EGFR中与Asp855形成直接氢键的配体。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号