当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Ionic Cu(II) and Co(II) DACH–Flufenamate Conjugate Complexes: Spectroscopic Characterization, Single X–Ray Studies and Cytotoxic Activity on Human Cancer Cell Lines
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-12-04 , DOI: 10.1002/slct.201802698 Huzaifa Yasir Khan 1 , Siffeen Zehra 1 , Sabiha Parveen 1 , Imtiyaz Yousuf 1 , Sartaj Tabassum 1 , Farukh Arjmand 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-12-04 , DOI: 10.1002/slct.201802698 Huzaifa Yasir Khan 1 , Siffeen Zehra 1 , Sabiha Parveen 1 , Imtiyaz Yousuf 1 , Sartaj Tabassum 1 , Farukh Arjmand 1
Affiliation
|
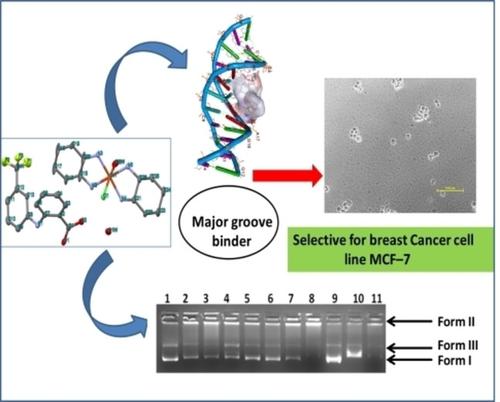
New ionic antitumor drug entities [{Cu(DACH)2(H2O)Cl}.(fluf)], 1 and [{Co(DACH)2(H2O)Cl}.(fluf)], 2 where DACH=1,2–diamminocyclohexane and fluf=flufenamate anion were prepared and characterized by spectroscopic studies and single X–ray crystallography. In order to evaluate the potential of these complexes to act as antitumor drug candidates, in vitro binding studies of 1 and 2 with ct–DNA have been carried out by biophysical methods which revealed electrostatic binding mode of these drug entities with ct–DNA preferably by external surface or groove binding mode contrary to other non–steroidal anti–inflammatory (NSAIDs) drugs which show intercalative binding. Comparative electron paramagnetic resonance (EPR) titrations were performed which revealed that there was no significant change in EPR spectra of complexes upon incubation with ct–DNA implicating that coordination geometry of metal ions does not alter. Validation of antitumor potential of 1 and 2 was done by cytotoxicity experiments against human cancer cell lines by Sulforhodamine B (SRB) assay. Complex 1 was active against all tested cell lines with an exceptionally low GI50 value=2.9 μg/ml against MCF–7 (breast cancer) cell line showing its preferential selectivity. On the contrary, complex 2 showed poor activity against all tested cancer cell lines.
中文翻译:

新型离子铜(II)和钴(II)DACH-氟草酸酯共轭复合物:光谱表征,单次X射线研究和对人类癌细胞系的细胞毒活性
新的离子型抗肿瘤药物实体[{Cu(DACH)2(H 2 O)Cl}。(fluf)],1和[{Co(DACH)2(H 2 O)Cl}。(fluf)],2,其中DACH制备了1,2,2-二氨基环己烷和fluf =氟苯甲酸酯阴离子,并通过光谱研究和单X射线晶体学进行了表征。为了评估这些复合物作为抗肿瘤药物候选物的潜力,对1和2进行了体外结合研究已经通过生物物理方法对ct-DNA进行了研究,揭示了这些药物实体与ct-DNA的静电结合模式,最好是通过外表面或凹槽结合模式进行,这与其他具有嵌入结合作用的非甾体类抗炎药(NSAIDs)相反。进行了比较电子顺磁共振(EPR)滴定,发现与ct–DNA孵育后,复合物的EPR谱图没有显着变化,这暗示金属离子的配位几何形状不会改变。1和2的抗肿瘤潜力的验证是通过对磺胺多巴酚B(SRB)的抗人癌细胞系的细胞毒性实验来完成的。复合物1对GI极低的所有受试细胞系均具有活性针对MCF-7(乳腺癌)细胞系的50值= 2.9μg/ ml,显示出其优先的选择性。相反,复合物2显示出对所有测试的癌细胞系的不良活性。
更新日期:2018-12-04
中文翻译:

新型离子铜(II)和钴(II)DACH-氟草酸酯共轭复合物:光谱表征,单次X射线研究和对人类癌细胞系的细胞毒活性
新的离子型抗肿瘤药物实体[{Cu(DACH)2(H 2 O)Cl}。(fluf)],1和[{Co(DACH)2(H 2 O)Cl}。(fluf)],2,其中DACH制备了1,2,2-二氨基环己烷和fluf =氟苯甲酸酯阴离子,并通过光谱研究和单X射线晶体学进行了表征。为了评估这些复合物作为抗肿瘤药物候选物的潜力,对1和2进行了体外结合研究已经通过生物物理方法对ct-DNA进行了研究,揭示了这些药物实体与ct-DNA的静电结合模式,最好是通过外表面或凹槽结合模式进行,这与其他具有嵌入结合作用的非甾体类抗炎药(NSAIDs)相反。进行了比较电子顺磁共振(EPR)滴定,发现与ct–DNA孵育后,复合物的EPR谱图没有显着变化,这暗示金属离子的配位几何形状不会改变。1和2的抗肿瘤潜力的验证是通过对磺胺多巴酚B(SRB)的抗人癌细胞系的细胞毒性实验来完成的。复合物1对GI极低的所有受试细胞系均具有活性针对MCF-7(乳腺癌)细胞系的50值= 2.9μg/ ml,显示出其优先的选择性。相反,复合物2显示出对所有测试的癌细胞系的不良活性。































 京公网安备 11010802027423号
京公网安备 11010802027423号