当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ATP-dependent membrane remodeling links EHD1 functions to endocytic recycling.
Nature Communications ( IF 14.7 ) Pub Date : 2018-12-05 , DOI: 10.1038/s41467-018-07586-z Raunaq Deo 1 , Manish S Kushwah 1 , Sukrut C Kamerkar 1 , Nagesh Y Kadam 2 , Srishti Dar 1 , Kavita Babu 2 , Anand Srivastava 3 , Thomas J Pucadyil 1
Nature Communications ( IF 14.7 ) Pub Date : 2018-12-05 , DOI: 10.1038/s41467-018-07586-z Raunaq Deo 1 , Manish S Kushwah 1 , Sukrut C Kamerkar 1 , Nagesh Y Kadam 2 , Srishti Dar 1 , Kavita Babu 2 , Anand Srivastava 3 , Thomas J Pucadyil 1
Affiliation
|
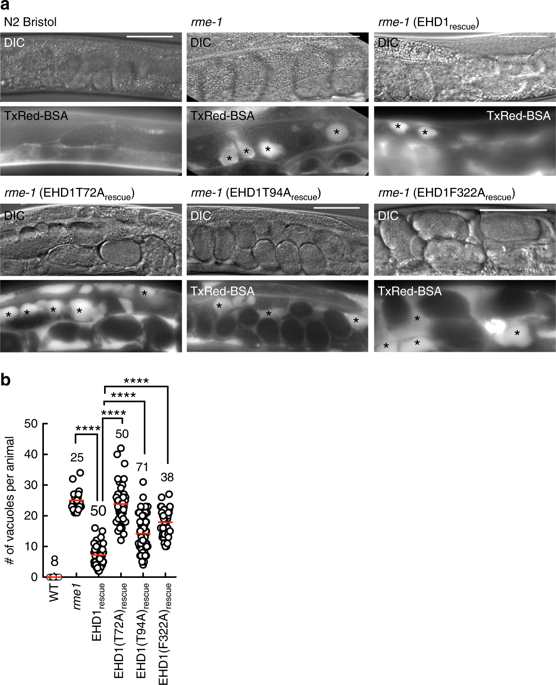
Endocytic and recycling pathways generate cargo-laden transport carriers by membrane fission. Classical dynamins, which generate transport carriers during endocytosis, constrict and cause fission of membrane tubes in response to GTP hydrolysis. Relatively, less is known about the ATP-binding Eps15-homology domain-containing protein1 (EHD1), a dynamin family member that functions at the endocytic-recycling compartment. Here, we show using cross complementation assays in C. elegans that EHD1's membrane binding and ATP hydrolysis activities are necessary for endocytic recycling. Further, we show that ATP-bound EHD1 forms membrane-active scaffolds that bulge tubular model membranes. ATP hydrolysis promotes scaffold self-assembly, causing the bulge to extend and thin down intermediate regions on the tube. On tubes below 25 nm in radius, such thinning leads to scission. Molecular dynamics simulations corroborate this scission pathway. Deletion of N-terminal residues causes defects in stable scaffolding, scission and endocytic recycling. Thus, ATP hydrolysis-dependent membrane remodeling links EHD1 functions to endocytic recycling.
中文翻译:

ATP 依赖性膜重塑将 EHD1 功能与内吞再循环联系起来。
内吞和回收途径通过膜裂变产生载有货物的运输载体。经典动力在内吞作用过程中产生运输载体,响应 GTP 水解而收缩并引起膜管裂变。相对而言,人们对 ATP 结合 Eps15 同源结构域蛋白 1 (EHD1) 知之甚少,EHD1 是在内吞循环室中发挥作用的动力家族成员。在这里,我们使用线虫中的交叉互补测定表明,EHD1 的膜结合和 ATP 水解活性对于内吞循环是必需的。此外,我们还发现 ATP 结合的 EHD1 形成膜活性支架,使管状模型膜凸出。 ATP 水解促进支架自组装,导致凸起延伸并变薄管上的中间区域。在半径低于 25 nm 的管上,这种变薄会导致断裂。分子动力学模拟证实了这种断裂途径。 N 末端残基的缺失会导致稳定支架、断裂和内吞再循环的缺陷。因此,ATP 水解依赖性膜重塑将 EHD1 功能与内吞再循环联系起来。
更新日期:2018-12-05
中文翻译:

ATP 依赖性膜重塑将 EHD1 功能与内吞再循环联系起来。
内吞和回收途径通过膜裂变产生载有货物的运输载体。经典动力在内吞作用过程中产生运输载体,响应 GTP 水解而收缩并引起膜管裂变。相对而言,人们对 ATP 结合 Eps15 同源结构域蛋白 1 (EHD1) 知之甚少,EHD1 是在内吞循环室中发挥作用的动力家族成员。在这里,我们使用线虫中的交叉互补测定表明,EHD1 的膜结合和 ATP 水解活性对于内吞循环是必需的。此外,我们还发现 ATP 结合的 EHD1 形成膜活性支架,使管状模型膜凸出。 ATP 水解促进支架自组装,导致凸起延伸并变薄管上的中间区域。在半径低于 25 nm 的管上,这种变薄会导致断裂。分子动力学模拟证实了这种断裂途径。 N 末端残基的缺失会导致稳定支架、断裂和内吞再循环的缺陷。因此,ATP 水解依赖性膜重塑将 EHD1 功能与内吞再循环联系起来。

































 京公网安备 11010802027423号
京公网安备 11010802027423号