当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational Molecular Engineering of Glutamate Dehydrogenases for Enhancing Asymmetric Reductive Amination of Bulky α‐Keto Acids
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-12-28 , DOI: 10.1002/adsc.201801251
Xinjian Yin 1 , Yayun Liu 1 , Lijun Meng 1 , Haisheng Zhou 1 , Jianping Wu 1 , Lirong Yang 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-12-28 , DOI: 10.1002/adsc.201801251
Xinjian Yin 1 , Yayun Liu 1 , Lijun Meng 1 , Haisheng Zhou 1 , Jianping Wu 1 , Lirong Yang 1
Affiliation
|
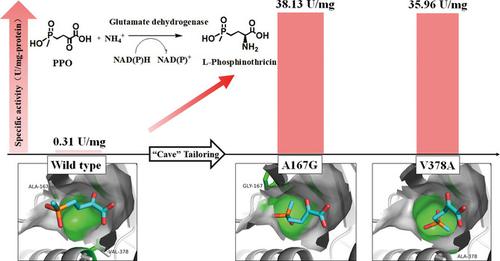
Glutamate dehydrogenases (GluDHs) are promising biocatalysts for the synthesis of chiral α‐amino acids by asymmetric reductive amination of α‐keto acids. However, their strict substrate specificity limits their applications. To address this problem, we developed a molecular engineering method for GluDHs that enhances the asymmetric reductive amination of bulky α‐keto acids. Based on rational design, a “cave” located in the active site pocket of PpGluDH (GluDH from Pseudomonas putida), which plays an essential role in substrate recognition, was tailored to facilitate the accepting of bulky substrates. Two mutants (A167G and V378A) were discovered to have significantly enhanced catalytic activity toward 2‐oxo‐4‐[(hydroxy)(methyl)phosphinyl]butyric acid (PPO) and several other bulky substrates. This molecular engineering method was then applied to ten other GluDHs from different sources and with different properties. All engineered GluDHs acquired substantial improvements in PPO‐oriented catalytic activity. The most efficient mutant of NADP+ (nicotinamide adenine dinucleotide phosphate)‐specific GluDHs showed up to 1820‐fold increased activity and the specific activity reached 111.02 U/mg‐protein. The NAD+ (nicotinamide adenine dinucleotide)‐specific GluDHs, which have no detectable wild type activity toward PPO, acquired a considerable level of activity (1.90–29.48 U/mg‐protein). In batch production of L‐phosphinothricin, these “cave‐tailored” GluDHs exhibited markedly improved catalytic efficiencies compared with their wild types and ee values of >99%. The space‐time yields (STY) varied from 818.16 to 1482.96 g ⋅ L−1 ⋅ d−1, suggesting potential practical applications of these mutants.
中文翻译:

谷氨酸脱氢酶的合理分子工程,以增强大体积α-酮酸的不对称还原胺化
谷氨酸脱氢酶(GluDHs)是通过α-酮酸的不对称还原胺化合成手性α-氨基酸的有前途的生物催化剂。但是,它们严格的底物特异性限制了它们的应用。为了解决这个问题,我们开发了一种用于GluDHs的分子工程方法,该方法可增强大体积α-酮酸的不对称还原胺化作用。基于合理的设计,“洞”位于Pp GluDH(恶臭假单胞菌的GluDH)的活性位点)在底物识别中起着至关重要的作用,经过了量身定制,以方便大体积底物的接受。发现两个突变体(A167G和V378A)对2-氧代-4-[[(羟基)(甲基)亚膦酰基]丁酸(PPO)和其他一些大的底物具有显着增强的催化活性。然后将该分子工程方法应用于来自不同来源和具有不同性质的十种其他GluDH。所有工程化的GluDHs在面向PPO的催化活性方面均取得了显着改善。最有效的NADP +(烟酰胺腺嘌呤二核苷酸磷酸)特异性GluDHs突变体的活性提高了1820倍,比活性达到111.02 U / mg-蛋白。NAD +(烟酰胺腺嘌呤二核苷酸)特异性GluDHs对PPO没有检测到野生型活性,但具有相当高的活性(1.90–29.48 U / mg-蛋白)。在批量生产L-膦丝菌素时,与野生型和ee值> 99%相比,这些“凹形” GluDHs的催化效率显着提高。从818.16到1482.96克⋅大号而变化的时空产率(STY)-1 ⋅d -1,这表明这些突变体的潜在的实际应用。
更新日期:2018-12-28
中文翻译:

谷氨酸脱氢酶的合理分子工程,以增强大体积α-酮酸的不对称还原胺化
谷氨酸脱氢酶(GluDHs)是通过α-酮酸的不对称还原胺化合成手性α-氨基酸的有前途的生物催化剂。但是,它们严格的底物特异性限制了它们的应用。为了解决这个问题,我们开发了一种用于GluDHs的分子工程方法,该方法可增强大体积α-酮酸的不对称还原胺化作用。基于合理的设计,“洞”位于Pp GluDH(恶臭假单胞菌的GluDH)的活性位点)在底物识别中起着至关重要的作用,经过了量身定制,以方便大体积底物的接受。发现两个突变体(A167G和V378A)对2-氧代-4-[[(羟基)(甲基)亚膦酰基]丁酸(PPO)和其他一些大的底物具有显着增强的催化活性。然后将该分子工程方法应用于来自不同来源和具有不同性质的十种其他GluDH。所有工程化的GluDHs在面向PPO的催化活性方面均取得了显着改善。最有效的NADP +(烟酰胺腺嘌呤二核苷酸磷酸)特异性GluDHs突变体的活性提高了1820倍,比活性达到111.02 U / mg-蛋白。NAD +(烟酰胺腺嘌呤二核苷酸)特异性GluDHs对PPO没有检测到野生型活性,但具有相当高的活性(1.90–29.48 U / mg-蛋白)。在批量生产L-膦丝菌素时,与野生型和ee值> 99%相比,这些“凹形” GluDHs的催化效率显着提高。从818.16到1482.96克⋅大号而变化的时空产率(STY)-1 ⋅d -1,这表明这些突变体的潜在的实际应用。































 京公网安备 11010802027423号
京公网安备 11010802027423号