Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Triphenylethylene- and Tetraphenylethylene-Functionalized 1,3-Bis(pyrrol-2-yl)squaraine Dyes: Synthesis, Aggregation-Caused Quenching to Aggregation-Induced Emission, and Thiol Detection
ACS Omega ( IF 3.7 ) Pub Date : 2018-12-03 00:00:00 , DOI: 10.1021/acsomega.8b02479
Ming Hui Chua 1 , Hui Zhou 1 , Ting Ting Lin 1 , Jishan Wu 2 , Jianwei Xu 1
ACS Omega ( IF 3.7 ) Pub Date : 2018-12-03 00:00:00 , DOI: 10.1021/acsomega.8b02479
Ming Hui Chua 1 , Hui Zhou 1 , Ting Ting Lin 1 , Jishan Wu 2 , Jianwei Xu 1
Affiliation
|
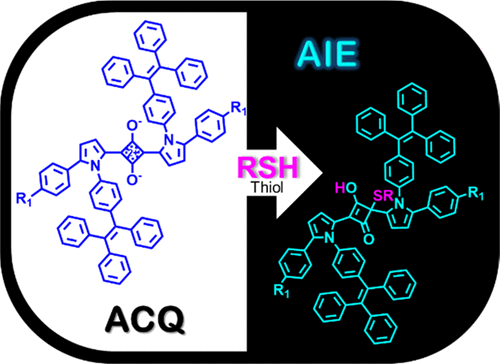
Three novel pairs of 1,3-bis(pyrrol-2-yl)squaraine dyes, N-alkylated SQ1a–1b, and N-phenylated SQ2a–2b in which triphenylethylene moieties functionalized at 5-position of pyrrole, as well as SQ3a–3b with tetraphenylethylene (TPE) moieties attached at N-position of pyrrole, were synthesized. All six dyes were found not to exhibit aggregation-induced emission (AIE) properties. Spectrophotometric studies showed that N-TPE-functionalized SQ3a–3b exhibited much larger molar extinction coefficients (ε: 1.36–2.14 × 105 M–1 cm–1) than 5,5′-triphenylethylene-functionalized SQ1a–2b (ε: 2.17–8.22 × 104 M–1 cm–1). Surprisingly, SQ2b showed a remarkable red-shifted maximum absorption (λmax: 723 vs 631–652 nm) compared to that of other squaraine dyes. All six squaraine dyes selectively responded to the addition of thiol-containing biomolecules, such as cysteine and gluthatione, with the disappearance of λmax in the near-infrared region in their respective absorption spectra. Interestingly, the thiolated species of SQ3a–3b were AIE active, with the characteristic AIE emission of TPE at λmax = 484–490 nm upon addition of water. Further thiol sensing on solid supports was examined, indicating the potential applications of TPE-functionalized squaraine dyes as bioprobes for the detection of important thiol-containing biomolecules, with a clear change from aggregation-caused quenching to AIE.
中文翻译:

三苯乙烯和四苯乙烯官能化的1,3-双(吡咯-2-基)方酸染料:合成,聚集猝灭到聚集诱导的发射和硫醇检测
三对新的1,3-双(吡咯-2-基)方酸染料,N-烷基化的SQ1a – 1b和N-苯基化的SQ2a – 2b,其中三苯基乙烯部分在吡咯的5位官能化,以及SQ3a –合成了在吡咯的N位连接有四苯基乙烯(TPE)部分的3b。发现所有六种染料均未表现出聚集诱导发射(AIE)特性。分光光度法研究表明,N -TPE官能化的SQ3a – 3b表现出更大的摩尔消光系数(ε:1.36–2.14×10 5M –1 cm –1)比5,5'-三苯乙烯官能化的SQ1a – 2b(ε:2.17–8.22×10 4 M –1 cm –1)。令人惊讶的是,与其他方酸类染料相比,SQ2b表现出了显着的红移最大吸收(λmax:723 vs 631–652 nm)。所有六种方酸菁染料对含巯基生物分子(例如半胱氨酸和谷胱甘肽)的添加做出选择性反应,其各自吸收光谱中的近红外区域中的λmax消失。有趣的是,SQ3a – 3b的硫醇化物种具有AIE活性,添加水后,TPE在λmax = 484–490 nm处具有特征性的AIE发射。检查了对固体支持物的进一步硫醇感测,表明TPE官能化的方酸染料作为生物探针在检测重要的含硫醇生物分子方面的潜在应用,从聚集引起的猝灭到AIE的明显变化。
更新日期:2018-12-03
中文翻译:

三苯乙烯和四苯乙烯官能化的1,3-双(吡咯-2-基)方酸染料:合成,聚集猝灭到聚集诱导的发射和硫醇检测
三对新的1,3-双(吡咯-2-基)方酸染料,N-烷基化的SQ1a – 1b和N-苯基化的SQ2a – 2b,其中三苯基乙烯部分在吡咯的5位官能化,以及SQ3a –合成了在吡咯的N位连接有四苯基乙烯(TPE)部分的3b。发现所有六种染料均未表现出聚集诱导发射(AIE)特性。分光光度法研究表明,N -TPE官能化的SQ3a – 3b表现出更大的摩尔消光系数(ε:1.36–2.14×10 5M –1 cm –1)比5,5'-三苯乙烯官能化的SQ1a – 2b(ε:2.17–8.22×10 4 M –1 cm –1)。令人惊讶的是,与其他方酸类染料相比,SQ2b表现出了显着的红移最大吸收(λmax:723 vs 631–652 nm)。所有六种方酸菁染料对含巯基生物分子(例如半胱氨酸和谷胱甘肽)的添加做出选择性反应,其各自吸收光谱中的近红外区域中的λmax消失。有趣的是,SQ3a – 3b的硫醇化物种具有AIE活性,添加水后,TPE在λmax = 484–490 nm处具有特征性的AIE发射。检查了对固体支持物的进一步硫醇感测,表明TPE官能化的方酸染料作为生物探针在检测重要的含硫醇生物分子方面的潜在应用,从聚集引起的猝灭到AIE的明显变化。


































 京公网安备 11010802027423号
京公网安备 11010802027423号