当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carbonate Adsorption to Ferrihydrite: Competitive Interaction with Phosphate for Use in Soil Systems
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-12-03 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00160 Juan C Mendez 1 , Tjisse Hiemstra 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-12-03 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00160 Juan C Mendez 1 , Tjisse Hiemstra 1
Affiliation
|
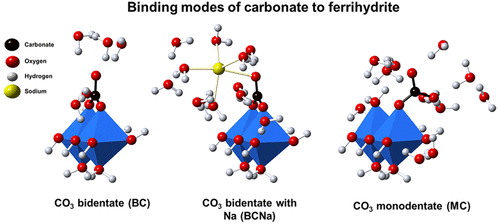
Carbonate (CO3) interacts with Fe-(hydr)oxide nanoparticles, affecting the availability and geochemical cycle of other important oxyanions in nature. Here, we studied the carbonate–phosphate interaction in closed systems with freshly prepared ferrihydrite (Fh), using batch experiments that cover a wide range of pH values, ionic strength, and CO3 and PO4 concentrations. The surface speciation of CO3 has been assessed by interpreting the ion competition with the Charge Distribution (CD) model, using CD coefficients derived from MO/DTF optimized geometries. Adsorption of CO3 occurs predominately via formation of bidentate inner-sphere complexes, either (≡FeO)2CO or (≡FeO)2CO··Na+. The latter complex is electrostatically promoted at high pH and in the presence of adsorbed PO4. Additionally, a minor complex is present at high CO3 loadings. The CD model, solely parametrized by measuring the pH-dependent PO4 adsorption as a function of the CO3 concentration, successfully predicts the CO3 adsorption to Fh in single-ion systems. The adsorption affinity of CO3 to Fh is higher than to goethite, particularly at high pH and CO3 loadings due to the enhanced formation (≡FeO)2CO··Na+. The PO4 adsorption isotherm in 0.5 M NaHCO3 can be well described, being relevant for assessing the reactive surface area of the natural oxide fraction with soil extractions and CD modeling. Additionally, we have evaluated the enhanced Fh solubility due to Fe(III)-CO3 complex formation and resolved a new species (Fe(CO3)2(OH)23−(aq)), which is dominant in closed systems at high pH. The measured solubility of our Fh agrees with the size-dependent solubility predicted using the surface Gibbs free energy of Fh.
中文翻译:

碳酸盐对水铁矿的吸附:与土壤系统中使用的磷酸盐的竞争性相互作用
碳酸盐 (CO 3 ) 与铁(氢)氧化物纳米颗粒相互作用,影响自然界中其他重要含氧阴离子的可用性和地球化学循环。在这里,我们使用涵盖广泛 pH 值、离子强度以及 CO 3和 PO 4浓度的批量实验,研究了封闭系统中新制备的水铁矿 (Fh) 的碳酸盐 - 磷酸盐相互作用。 CO 3的表面形态通过使用从 MO/DTF 优化几何形状导出的 CD 系数解释与电荷分布 (CD) 模型的离子竞争来评估。 CO 3的吸附主要通过形成二齿内球复合物((≡FeO) 2 CO 或(≡FeO) 2 CO··Na + )而发生。后一种络合物在高pH值和吸附的PO 4存在下被静电促进。此外,在高CO 3负载量下存在少量络合物。 CD 模型仅通过测量 pH 依赖性 PO 4吸附作为 CO 3浓度的函数进行参数化,成功地预测了单离子系统中 Fh 的 CO 3吸附。 CO 3对Fh 的吸附亲和力高于对针铁矿的吸附亲和力,特别是在高pH 值和CO 3负载量下,由于(≡FeO) 2 CO··Na +的形成增强。可以很好地描述 0.5 M NaHCO 3中的 PO 4吸附等温线,这与通过土壤提取和 CD 建模评估自然氧化物部分的反应表面积相关。 此外,我们还评估了由于 Fe(III)-CO 3络合物形成而增强的 Fh 溶解度,并解析了一种新物质 (Fe(CO 3 ) 2 (OH) 2 3− (aq)),该物质在高pH值。我们测量的 Fh 溶解度与使用 Fh 表面吉布斯自由能预测的尺寸依赖性溶解度一致。
更新日期:2018-12-03
中文翻译:

碳酸盐对水铁矿的吸附:与土壤系统中使用的磷酸盐的竞争性相互作用
碳酸盐 (CO 3 ) 与铁(氢)氧化物纳米颗粒相互作用,影响自然界中其他重要含氧阴离子的可用性和地球化学循环。在这里,我们使用涵盖广泛 pH 值、离子强度以及 CO 3和 PO 4浓度的批量实验,研究了封闭系统中新制备的水铁矿 (Fh) 的碳酸盐 - 磷酸盐相互作用。 CO 3的表面形态通过使用从 MO/DTF 优化几何形状导出的 CD 系数解释与电荷分布 (CD) 模型的离子竞争来评估。 CO 3的吸附主要通过形成二齿内球复合物((≡FeO) 2 CO 或(≡FeO) 2 CO··Na + )而发生。后一种络合物在高pH值和吸附的PO 4存在下被静电促进。此外,在高CO 3负载量下存在少量络合物。 CD 模型仅通过测量 pH 依赖性 PO 4吸附作为 CO 3浓度的函数进行参数化,成功地预测了单离子系统中 Fh 的 CO 3吸附。 CO 3对Fh 的吸附亲和力高于对针铁矿的吸附亲和力,特别是在高pH 值和CO 3负载量下,由于(≡FeO) 2 CO··Na +的形成增强。可以很好地描述 0.5 M NaHCO 3中的 PO 4吸附等温线,这与通过土壤提取和 CD 建模评估自然氧化物部分的反应表面积相关。 此外,我们还评估了由于 Fe(III)-CO 3络合物形成而增强的 Fh 溶解度,并解析了一种新物质 (Fe(CO 3 ) 2 (OH) 2 3− (aq)),该物质在高pH值。我们测量的 Fh 溶解度与使用 Fh 表面吉布斯自由能预测的尺寸依赖性溶解度一致。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号