Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Felis catus papillomavirus type-2 E6 binds to E6AP, promotes E6AP/p53 binding and enhances p53 proteasomal degradation.
Scientific Reports ( IF 3.8 ) Pub Date : 2018-Dec-03 , DOI: 10.1038/s41598-018-35723-7 Gennaro Altamura , Karen Power , Manuela Martano , Barbara degli Uberti , Giorgio Galiero , Giovanna De Luca , Paola Maiolino , Giuseppe Borzacchiello
Scientific Reports ( IF 3.8 ) Pub Date : 2018-Dec-03 , DOI: 10.1038/s41598-018-35723-7 Gennaro Altamura , Karen Power , Manuela Martano , Barbara degli Uberti , Giorgio Galiero , Giovanna De Luca , Paola Maiolino , Giuseppe Borzacchiello
|
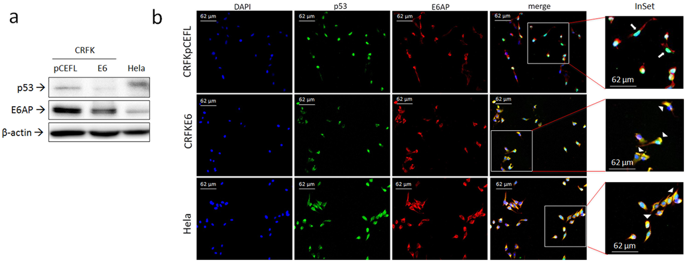
E6 from high risk human papillomaviruses (HR HPVs) promotes ubiquitination and degradation of p53 tumour suppressor by mediating its binding to ubiquitin ligase E6AP in a ternary complex, contributing to cell transformation in cervical cancer. We have previously shown that Felis catus papillomavirus type -2 (FcaPV-2) E6 is expressed in feline squamous cell carcinoma (SCC) and displays the ability to bind p53 and decrease its protein levels in transfected CRFK cells. However, the mechanism of p53 downregulation has not yet been characterized. Here we show that FcaPV-2 E6 bound to E6AP, which in turn was bound by p53 exclusively in cells expressing the viral oncoprotein (CRFKE6). Furthermore, p53 was highly poly-ubiquitinated and underwent accumulation upon E6AP gene knockdown in CRFKE6. Half-life experiments and proteasome inhibition treatments indicated that down-regulation of p53 protein in CRFKE6 was due to accelerated proteasomal degradation. E6AP/p53 binding was also demonstrated in two feline SCC cell lines expressing FcaPV-2 E6, where p53 protein levels and poly-ubiquitination degree were proportional to E6 mRNA levels. The data obtained in both artificial and spontaneous in vitro models suggest that FcaPV-2 E6 degrades p53 through a molecular mechanism similar to HR HPVs, possibly contributing to the development of feline SCC.
中文翻译:

2型猫猫乳头瘤病毒E6与E6AP结合,促进E6AP / p53结合并增强p53蛋白酶体降解。
高危人类乳头瘤病毒(HR HPV)产生的E6通过介导三态复合体中泛素连接酶E6AP的结合,促进p53肿瘤抑制因子的泛素化和降解,从而促进宫颈癌的细胞转化。先前我们已经表明猫猫乳头瘤病毒-2型(FcaPV-2)E6在猫鳞状细胞癌(SCC)中表达,并且在转染的CRFK细胞中显示出结合p53并降低其蛋白水平的能力。但是,p53下调的机制尚未被鉴定。在这里,我们显示FcaPV-2 E6与E6AP结合,而E6AP仅在表达病毒癌蛋白(CRFKE6)的细胞中被p53结合。此外,p53是高度多聚泛素化的,并在CRFKE6中的E6AP基因敲低后进行积累。半衰期实验和蛋白酶体抑制处理表明,CRFKE6中p53蛋白的下调是由于蛋白酶体降解加速所致。在表达FcaPV-2 E6的两种猫SCC细胞系中也证实了E6AP / p53的结合,其中p53蛋白水平和多聚泛素化程度与E6 mRNA水平成正比。在人工和自发体外模型中获得的数据表明,FcaPV-2 E6通过类似于HR HPV的分子机制降解p53,可能有助于猫SCC的发展。
更新日期:2018-12-03
中文翻译:

2型猫猫乳头瘤病毒E6与E6AP结合,促进E6AP / p53结合并增强p53蛋白酶体降解。
高危人类乳头瘤病毒(HR HPV)产生的E6通过介导三态复合体中泛素连接酶E6AP的结合,促进p53肿瘤抑制因子的泛素化和降解,从而促进宫颈癌的细胞转化。先前我们已经表明猫猫乳头瘤病毒-2型(FcaPV-2)E6在猫鳞状细胞癌(SCC)中表达,并且在转染的CRFK细胞中显示出结合p53并降低其蛋白水平的能力。但是,p53下调的机制尚未被鉴定。在这里,我们显示FcaPV-2 E6与E6AP结合,而E6AP仅在表达病毒癌蛋白(CRFKE6)的细胞中被p53结合。此外,p53是高度多聚泛素化的,并在CRFKE6中的E6AP基因敲低后进行积累。半衰期实验和蛋白酶体抑制处理表明,CRFKE6中p53蛋白的下调是由于蛋白酶体降解加速所致。在表达FcaPV-2 E6的两种猫SCC细胞系中也证实了E6AP / p53的结合,其中p53蛋白水平和多聚泛素化程度与E6 mRNA水平成正比。在人工和自发体外模型中获得的数据表明,FcaPV-2 E6通过类似于HR HPV的分子机制降解p53,可能有助于猫SCC的发展。






























 京公网安备 11010802027423号
京公网安备 11010802027423号