当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Demystifying Cp2Ti(H)Cl and Its Enigmatic Role in the Reactions of Epoxides with Cp2TiCl
Organometallics ( IF 2.5 ) Pub Date : 2018-11-30 , DOI: 10.1021/acs.organomet.8b00793
Jonathan Gordon 1 , Sven Hildebrandt 2 , Kendra R. Dewese 1 , Sven Klare 2 , Andreas Gansäuer 2 , T. V. RajanBabu 1 , William A. Nugent 1
Organometallics ( IF 2.5 ) Pub Date : 2018-11-30 , DOI: 10.1021/acs.organomet.8b00793
Jonathan Gordon 1 , Sven Hildebrandt 2 , Kendra R. Dewese 1 , Sven Klare 2 , Andreas Gansäuer 2 , T. V. RajanBabu 1 , William A. Nugent 1
Affiliation
|
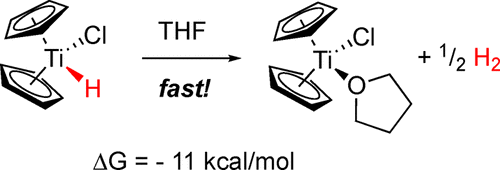
The role of Cp2Ti(H)Cl in the reactions of Cp2TiCl with trisubstituted epoxides has been investigated in a combined experimental and computational study. Although Cp2Ti(H)Cl has generally been regarded as a robust species, its decomposition to Cp2TiCl and molecular hydrogen was found to be exothermic (ΔG = −11 kcal/mol when the effects of THF solvation are considered). In laboratory studies, Cp2Ti(H)Cl was generated using the reaction of 1,2-epoxy-1-methylcyclohexane with Cp2TiCl as a model. Rapid evolution of hydrogen gas was demonstrated, indicating that Cp2Ti(H)Cl is indeed a thermally unstable molecule, which undergoes intermolecular reductive elimination of hydrogen under the reaction conditions. The stoichiometry of the reaction (Cp2TiCl:epoxide = 1:1) and the quantity of hydrogen produced (1 mol per 2 mol of epoxide) is consistent with this assertion. The diminished yield of allylic alcohol from these reactions under the conditions of protic versus aprotic catalysis can be understood in terms of the predominant titanium(III) present in solution. Under the conditions of protic catalysis, Cp2TiCl complexes with collidine hydrochloride and the titanium(III) center is less available for “cross-disproportionation” with carbon-centered radicals; this leads to byproducts from radical capture by hydrogen atom transfer, resulting in a saturated alcohol.
中文翻译:

Cp 2 Ti(H)Cl的去神秘化及其在环氧化合物与Cp 2 TiCl反应中的神秘作用
在组合的实验和计算研究中,已经研究了Cp 2 Ti(H)Cl在Cp 2 TiCl与三取代环氧化物的反应中的作用。尽管通常将Cp 2 Ti(H)Cl视为强壮物种,但发现它分解为Cp 2 TiCl和分子氢是放热的(考虑到THF溶剂化的影响,ΔG = -11 kcal / mol)。在实验室研究中,使用1,2-环氧-1-甲基环己烷与Cp 2 TiCl作为模型生成了Cp 2 Ti(H)Cl 。证明了氢气的快速释放,表明Cp 2Ti(H)Cl确实是一种热不稳定分子,在反应条件下会进行分子间还原性氢消除。反应的化学计量(Cp 2 TiCl:环氧化物= 1:1)和产生的氢气量(每2摩尔环氧化物1摩尔)与此说法相符。在质子对非质子催化下,这些反应中烯丙醇的产率降低,可以理解为存在于溶液中的主要钛(III)。在质子催化条件下,Cp 2TiCl与可力丁盐酸盐和钛(III)中心的络合物与以碳为中心的自由基“交叉歧化”的可能性较小。这会导致自由基通过氢原子转移而捕获副产物,从而生成饱和醇。
更新日期:2018-12-01
中文翻译:

Cp 2 Ti(H)Cl的去神秘化及其在环氧化合物与Cp 2 TiCl反应中的神秘作用
在组合的实验和计算研究中,已经研究了Cp 2 Ti(H)Cl在Cp 2 TiCl与三取代环氧化物的反应中的作用。尽管通常将Cp 2 Ti(H)Cl视为强壮物种,但发现它分解为Cp 2 TiCl和分子氢是放热的(考虑到THF溶剂化的影响,ΔG = -11 kcal / mol)。在实验室研究中,使用1,2-环氧-1-甲基环己烷与Cp 2 TiCl作为模型生成了Cp 2 Ti(H)Cl 。证明了氢气的快速释放,表明Cp 2Ti(H)Cl确实是一种热不稳定分子,在反应条件下会进行分子间还原性氢消除。反应的化学计量(Cp 2 TiCl:环氧化物= 1:1)和产生的氢气量(每2摩尔环氧化物1摩尔)与此说法相符。在质子对非质子催化下,这些反应中烯丙醇的产率降低,可以理解为存在于溶液中的主要钛(III)。在质子催化条件下,Cp 2TiCl与可力丁盐酸盐和钛(III)中心的络合物与以碳为中心的自由基“交叉歧化”的可能性较小。这会导致自由基通过氢原子转移而捕获副产物,从而生成饱和醇。

































 京公网安备 11010802027423号
京公网安备 11010802027423号