当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid Identification of Disulfide Bonds and Cysteine-Related Variants in an IgG1 Knob-into-Hole Bispecific Antibody Enhanced by Machine Learning.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-12-01 00:00:00 , DOI: 10.1021/acs.analchem.8b04071 Jordan J Baker 1 , Dana McDaniel 1 , David Cain 1 , Paula Lee Tao 1 , Charlene Li 1 , Yuting Huang 1 , Hongbin Liu 1 , Judith Zhu-Shimoni 1 , Milady Niñonuevo 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-12-01 00:00:00 , DOI: 10.1021/acs.analchem.8b04071 Jordan J Baker 1 , Dana McDaniel 1 , David Cain 1 , Paula Lee Tao 1 , Charlene Li 1 , Yuting Huang 1 , Hongbin Liu 1 , Judith Zhu-Shimoni 1 , Milady Niñonuevo 1
Affiliation
|
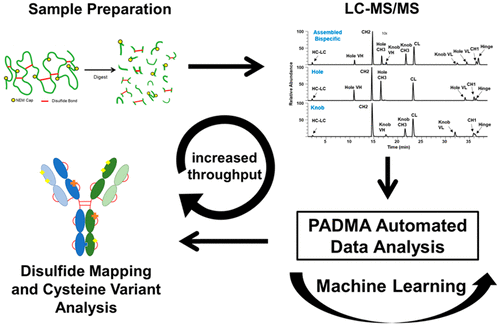
Bispecific antibodies are regarded as the next generation of therapeutic modalities as they can simultaneously bind multiple targets, increasing the efficacy of treatments for several diseases and opening up previously unattainable treatment designs. Linking two half antibodies to form the knob-into-hole bispecific antibody requires an additional in vitro assembly step, starting with reduction of the antibodies and then reoxidization. Analysis of the disulfide bonds (DSBs) is vital to ensuring the correct assembly, stability, and higher-order structures of these important biomolecules because incorrect disulfide bond formation and/or presence of cysteine-related post-translational modifications can cause a loss of biological activity or even elicit an immune response from the host. Despite advancements in analytical methods, characterization of cysteine forms remains technically challenging and time-consuming. Herein, we report the development of an improved nonreduced peptide map method coupled with machine learning to enable rapid identification of disulfide bonds and cysteine-related variants in an IgG1 knob-into-hole bispecific antibody. The enhanced method offers a fast, consistent, and accurate workflow in mapping-out expected disulfide bonds in both half antibodies and bispecific antibodies and identifying cysteine-related modifications. Comparisons between two versions of the bispecific antibody molecule and analysis of stressed samples were also accomplished, indicating this method can be utilized to identify alterations originating from bioprocess changes and to determine the impact of assembly and postassembly stress conditions to product quality.
中文翻译:

通过机器学习增强对IgG1旋钮入孔双特异性抗体中二硫键和半胱氨酸相关变体的快速鉴定。
双特异性抗体被认为是下一代的治疗方式,因为它们可以同时结合多个靶标,从而提高了针对多种疾病的治疗功效,并开启了以前无法实现的治疗设计。连接两个半抗体以形成钮入孔双特异性抗体需要额外的体外组装步骤,首先还原抗体,然后再氧化。二硫键(DSB)的分析对于确保这些重要生物分子的正确组装,稳定性和高阶结构至关重要,因为不正确的二硫键形成和/或与半胱氨酸相关的翻译后修饰的存在会导致生物学损失。活性甚至引起宿主的免疫反应。尽管分析方法取得了进步,但是半胱氨酸形式的表征在技术上仍然具有挑战性和耗时。在本文中,我们报告了一种改进的非还原肽图方法的发展,该方法与机器学习相结合,能够快速识别IgG1旋入孔双特异性抗体中的二硫键和半胱氨酸相关变体。增强的方法可提供快速,一致,准确的工作流程,以绘制出半抗体和双特异性抗体中的预期二硫键,并鉴定与半胱氨酸相关的修饰。还完成了两个版本的双特异性抗体分子之间的比较以及对应激样品的分析,表明该方法可用于鉴定源自生物过程变化的变化,并确定组装和组装后应激条件对产品质量的影响。
更新日期:2018-12-01
中文翻译:

通过机器学习增强对IgG1旋钮入孔双特异性抗体中二硫键和半胱氨酸相关变体的快速鉴定。
双特异性抗体被认为是下一代的治疗方式,因为它们可以同时结合多个靶标,从而提高了针对多种疾病的治疗功效,并开启了以前无法实现的治疗设计。连接两个半抗体以形成钮入孔双特异性抗体需要额外的体外组装步骤,首先还原抗体,然后再氧化。二硫键(DSB)的分析对于确保这些重要生物分子的正确组装,稳定性和高阶结构至关重要,因为不正确的二硫键形成和/或与半胱氨酸相关的翻译后修饰的存在会导致生物学损失。活性甚至引起宿主的免疫反应。尽管分析方法取得了进步,但是半胱氨酸形式的表征在技术上仍然具有挑战性和耗时。在本文中,我们报告了一种改进的非还原肽图方法的发展,该方法与机器学习相结合,能够快速识别IgG1旋入孔双特异性抗体中的二硫键和半胱氨酸相关变体。增强的方法可提供快速,一致,准确的工作流程,以绘制出半抗体和双特异性抗体中的预期二硫键,并鉴定与半胱氨酸相关的修饰。还完成了两个版本的双特异性抗体分子之间的比较以及对应激样品的分析,表明该方法可用于鉴定源自生物过程变化的变化,并确定组装和组装后应激条件对产品质量的影响。

































 京公网安备 11010802027423号
京公网安备 11010802027423号