当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-Catalyzed Coupling Reactions of Cyclobutanone Oxime Esters with Sulfur Nucleophiles at Room Temperature
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-11-30 00:00:00 , DOI: 10.1021/acs.joc.8b02707 Mingchuang He 1 , Zhaohua Yan 1 , Fuyuan Zhu 1 , Sen Lin 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-11-30 00:00:00 , DOI: 10.1021/acs.joc.8b02707 Mingchuang He 1 , Zhaohua Yan 1 , Fuyuan Zhu 1 , Sen Lin 1
Affiliation
|
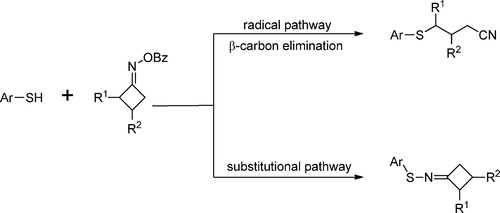
A copper-catalyzed iminyl radical-mediated C–C bond cleavage/cross-coupling tandem reaction of cyclobutanone oxime esters with aryl thiols in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) at room temperature was developed, and aryl cyanopropyl sulfides were smoothly synthesized in 20–88% yields. By altering the copper reagent and the molar ratio of cyclobutanone oxime ester/aryl thiol/DBU, substitutional product N-arylthio cyclobutanone imines were selectively generated in 50–91% yields. Using this protocol, C–S bond and N–S bond formations using aryl thiols as sulfur sources were realized under very mild conditions without the use of photocatalysis and electrocatalysis techniques.
中文翻译:

室温下铜催化环丁酮肟酯与硫亲核剂的偶联反应
在室温下1,8-二氮杂双环[5.4.0]十一烷基-7-烯(DBU)存在下,环丁酮肟酯与芳基硫醇的铜催化亚氨基自由基介导的CC键断裂/交叉偶联串联反应随着温度的升高,芳基氰基丙基硫醚以20–88%的收率顺利合成。通过改变铜试剂和环丁酮肟酯/芳基硫醇/ DBU的摩尔比,选择性生成N-芳硫基环丁酮亚胺的替代产物,收率为50-91%。使用该方案,在非常温和的条件下无需使用光催化和电催化技术即可实现使用芳基硫醇作为硫源的C–S键和N–S键的形成。
更新日期:2018-11-30
中文翻译:

室温下铜催化环丁酮肟酯与硫亲核剂的偶联反应
在室温下1,8-二氮杂双环[5.4.0]十一烷基-7-烯(DBU)存在下,环丁酮肟酯与芳基硫醇的铜催化亚氨基自由基介导的CC键断裂/交叉偶联串联反应随着温度的升高,芳基氰基丙基硫醚以20–88%的收率顺利合成。通过改变铜试剂和环丁酮肟酯/芳基硫醇/ DBU的摩尔比,选择性生成N-芳硫基环丁酮亚胺的替代产物,收率为50-91%。使用该方案,在非常温和的条件下无需使用光催化和电催化技术即可实现使用芳基硫醇作为硫源的C–S键和N–S键的形成。































 京公网安备 11010802027423号
京公网安备 11010802027423号