Redox Biology ( IF 10.7 ) Pub Date : 2018-11-29 , DOI: 10.1016/j.redox.2018.11.021
Aleksandr E. Vendrov , Arihiro Sumida , Chandrika Canugovi , Andrey Lozhkin , Takayuki Hayami , Nageswara R. Madamanchi , Marschall S. Runge
|
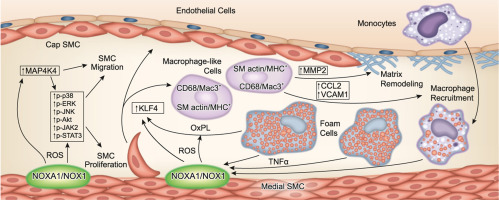
Increased reactive oxygen species (ROS) production and inflammation are key factors in the pathogenesis of atherosclerosis. We previously reported that NOX activator 1 (NOXA1) is the critical functional homolog of p67phox for NADPH oxidase activation in mouse vascular smooth muscle cells (VSMC). Here we investigated the effects of systemic and SMC-specific deletion of Noxa1 on VSMC phenotype during atherogenesis in mice.
Neointimal hyperplasia following endovascular injury was lower in Noxa1-deficient mice versus the wild-type following endovascular injury. Noxa1 deletion in Apoe-/- or Ldlr-/- mice fed a Western diet showed 50% reduction in vascular ROS and 30% reduction in aortic atherosclerotic lesion area and aortic sinus lesion volume (P < 0.01). SMC-specific deletion of Noxa1 in Apoe-/- mice (Noxa1SMC-/-/Apoe-/-) similarly decreased vascular ROS levels and atherosclerotic lesion size. TNFα-induced ROS generation, proliferation and migration were significantly attenuated in Noxa1-deficient versus wild-type VSMC. Immunofluorescence analysis of atherosclerotic lesions showed a significant decrease in cells positive for CD68 and myosin11 (22% versus 9%) and Mac3 and α-actin (17% versus 5%) in the Noxa1SMC-/-/Apoe-/- versus Apoe-/- mice. The expression of transcription factor KLF4, a modulator of VSMC phenotype, and its downstream targets – VCAM1, CCL2, and MMP2 – were significantly reduced in the lesions of Noxa1SMC-/-/Apoe-/- versus Apoe-/- mice as well as in oxidized phospholipids treated Noxa1SMC-/- versus wild-type VSMC.
Our data support an important role for NOXA1-dependent NADPH oxidase activity in VSMC plasticity during restenosis and atherosclerosis, augmenting VSMC proliferation and migration and KLF4-mediated transition to macrophage-like cells, plaque inflammation, and expansion.
中文翻译:

依赖NOXA1的NADPH氧化酶在动脉粥样硬化过程中调节氧化还原信号和血管平滑肌细胞表型
活性氧(ROS)产生和炎症的增加是动脉粥样硬化发病机理中的关键因素。我们以前报道过,NOX激活剂1(NOXA1)是p67phox在小鼠血管平滑肌细胞(VSMC)中对NADPH氧化酶激活的关键功能同源物。在这里,我们研究了在小鼠动脉粥样硬化过程中Noxa1全身性和SMC特异性缺失对VSMC表型的影响。
Noxa1缺陷小鼠的血管内损伤后新内膜增生低于血管内损伤后的野生型。用西方饮食喂养的Apoe -/-或Ldlr -/-小鼠中Noxa1缺失显示血管ROS减少50%,主动脉粥样硬化病变面积和主动脉窦病变体积减少30%(P <0.01)。的SMC-特异性缺失Noxa1在APOE / - -小鼠(Noxa1 SMC - / - / APOE - / -)同样降低了血管ROS水平和动脉粥样硬化病变的大小。TNFα诱导的ROS的产生,增殖和迁移在Noxa1缺陷型和野生型VSMC中显着减弱。动脉粥样硬化病变的免疫荧光分析显示,Noxa1 SMC-/- / Apoe -/-与Apoe相比,CD68和myosin11阳性细胞的细胞显着减少(22%对9%)以及Mac3和α-actin(17%对5%)。-/-老鼠。在Noxa1 SMC-/- / Apoe -/-的病变中,VSMC表型的调节因子转录因子KLF4的表达及其下游靶标VCAM1,CCL2和MMP2显着降低。与Apoe -/-小鼠相比,以及在氧化磷脂中处理的Noxa1 SMC-/-与野生型VSMC相比。
我们的数据支持NOXA1依赖性NADPH氧化酶活性在再狭窄和动脉粥样硬化过程中在VSMC可塑性中的重要作用,增加VSMC的增殖和迁移以及KLF4介导的向巨噬细胞样细胞的过渡,斑块炎症和扩张。

































 京公网安备 11010802027423号
京公网安备 11010802027423号