当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal-Free Direct Construction of 2-(Oxazol-5-yl)phenols from N-Phenoxyamides and Alkynylbenziodoxolones via Sequential [3,3]-Rearrangement/Cyclization
Organic Letters ( IF 4.9 ) Pub Date : 2018-11-29 00:00:00 , DOI: 10.1021/acs.orglett.8b03427 Ming Li 1 , Jia-Hui Wang 1 , Wei Li 1 , Li-Rong Wen 1
Organic Letters ( IF 4.9 ) Pub Date : 2018-11-29 00:00:00 , DOI: 10.1021/acs.orglett.8b03427 Ming Li 1 , Jia-Hui Wang 1 , Wei Li 1 , Li-Rong Wen 1
Affiliation
|
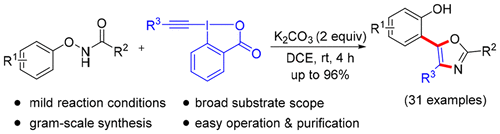
A mild and straightforward synthetic protocol for the construction of 2-(oxazol-5-yl)phenol derivatives promoted by K2CO3 from N-phenoxyamides and alkynylbenziodoxolones at room temperature has been developed. Importantly, this protocol involves a tandem sequence that includes [3,3]-rearrangement/alkylidene carbene insertion/Michael addition/cyclization. The metal-free conditions, broad substrate scope, and simple execution make this novel protocol very attractive.
中文翻译:

通过顺序[3,3]-重排/环化反应从N-苯氧基酰胺和炔基苯并恶唑啉酮中无金属直接构建2-(Ozazol-5-yl)苯酚
已经开发了一种温和简单的合成方案,用于在室温下构建由N-苯氧基酰胺和炔基苯并恶唑烷酮由K 2 CO 3促进的2-(恶唑-5-基)苯酚衍生物。重要的是,该协议涉及一个串联序列,该串联序列包括[3,3]重排/亚烷基卡宾插入/迈克尔加成/环化。无金属的条件,广泛的基板范围和简单的执行方式使这种新颖的协议非常有吸引力。
更新日期:2018-11-29
中文翻译:

通过顺序[3,3]-重排/环化反应从N-苯氧基酰胺和炔基苯并恶唑啉酮中无金属直接构建2-(Ozazol-5-yl)苯酚
已经开发了一种温和简单的合成方案,用于在室温下构建由N-苯氧基酰胺和炔基苯并恶唑烷酮由K 2 CO 3促进的2-(恶唑-5-基)苯酚衍生物。重要的是,该协议涉及一个串联序列,该串联序列包括[3,3]重排/亚烷基卡宾插入/迈克尔加成/环化。无金属的条件,广泛的基板范围和简单的执行方式使这种新颖的协议非常有吸引力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号