当前位置:
X-MOL 学术
›
Appl. Catal. B Environ. Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Supported H3PW12O40 for 2-propanol (photo-assisted) catalytic dehydration in gas-solid regime: The role of the support and of the pseudo-liquid phase in the (photo)activity
Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2016-03-06 18:53:42 Elisa I. García-López, Giuseppe Marcì, Francesca Rita Pomilla, Aleksandra Kirpsza, Anna Micek-Ilnicka, Leonardo Palmisano
Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2016-03-06 18:53:42 Elisa I. García-López, Giuseppe Marcì, Francesca Rita Pomilla, Aleksandra Kirpsza, Anna Micek-Ilnicka, Leonardo Palmisano
|
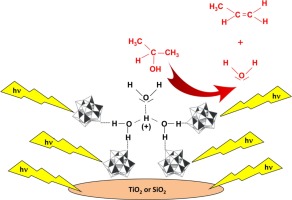
Catalytic and photocatalytic 2-propanol dehydration was carried out by using a supported Keggin heteropolyacid H3PW12O40 (PW12). Binary materials were prepared by impregnation and/or solvothermal treatment by using commercial supports: SiO2 (Mallinckrodt), TiO2 (Evonik P25) and multiwall carbon nanotubes (Sunnano) or home solvothermically prepared SiO2 and TiO2. All the materials have been characterized by X-ray diffraction (XRD), scanning electron microscopy observations (SEM) coupled with EDX microanalysis, specific surface area measurements, diffuse reflectance spectroscopy (DRS), FTIR and Raman spectroscopy. (Photo)catalytic 2-propanol dehydration was studied in gas-solid regime by using a continuous (photo)reactor working at atmospheric pressure and 80°C. FTIR spectra of the gas-phase over the PW12-support composites, where 2-propanol had been previously adsorbed, were recorded. Propene and diisopropyl ether were the main reaction products. For the continuous photo-assisted runs the reactor was also illuminated with UV light. The apparent activation energy of 2-propanol catalytic and photocatalytic dehydration was determined in the range 60–120°C. The irradiance increased significantly the dehydration reaction rate. Important differences were observed between the different supported materials. The Keggin heteropolyacid species played a key role both for the catalytic and the photo-assisted catalytic reactions; in fact, the acidity of the cluster accounts for the catalytic role, whereas both the acidity of the cluster and the oxidant ability of PW12 were responsible for the increase of the reaction rate of the photo-assisted catalytic reaction. Moreover, the photo-generated electrons on the solid semiconductor conduction band can account for a further increase of the reaction rate, when the heteropolyacid was supported on TiO2. Formation of a pseudo-liquid phase played an important role to determine the (photo)activity.
中文翻译:

用于气固状态下2-丙醇(光辅助)催化脱水的负载型H3PW12O40:载体和假液相在(光)活性中的作用
通过使用负载的Keggin杂多酸H 3 PW 12 O 40(PW 12)进行催化和光催化的2-丙醇脱水。二元材料是通过使用商业载体进行浸渍和/或溶剂热处理制得的:SiO 2(Mallinckrodt),TiO 2(Evonik P25)和多壁碳纳米管(Sunnano)或自制的热解法制备的SiO 2和TiO 2。所有材料的特征均在于X射线衍射(XRD),扫描电子显微镜观察(SEM)以及EDX显微分析,比表面积测量,漫反射光谱(DRS),FTIR和拉曼光谱。通过使用在大气压力和80°C下工作的连续(光)反应器,在气固状态下研究了(光)催化2-丙醇的脱水反应。PW 12上气相的FTIR光谱记录了先前已吸附了2-丙醇的β-载体复合材料。丙烯和二异丙醚是主要反应产物。对于连续的光辅助运行,反应器也用紫外线照射。在60–120°C范围内确定了2-丙醇催化和光催化脱水的表观活化能。辐照度显着提高了脱水反应速率。在不同的支撑材料之间观察到重要差异。Keggin杂多酸物质在催化和光辅助催化反应中都起着关键作用。实际上,簇的酸度起催化作用,而簇的酸度和PW 12的氧化能力负责光辅助催化反应的反应速率的增加。此外,当将杂多酸负载在TiO 2上时,在固体半导体导带上的光生电子可以说明反应速率的进一步提高。假液相的形成对确定(光)活性起重要作用。
更新日期:2016-03-07
中文翻译:

用于气固状态下2-丙醇(光辅助)催化脱水的负载型H3PW12O40:载体和假液相在(光)活性中的作用
通过使用负载的Keggin杂多酸H 3 PW 12 O 40(PW 12)进行催化和光催化的2-丙醇脱水。二元材料是通过使用商业载体进行浸渍和/或溶剂热处理制得的:SiO 2(Mallinckrodt),TiO 2(Evonik P25)和多壁碳纳米管(Sunnano)或自制的热解法制备的SiO 2和TiO 2。所有材料的特征均在于X射线衍射(XRD),扫描电子显微镜观察(SEM)以及EDX显微分析,比表面积测量,漫反射光谱(DRS),FTIR和拉曼光谱。通过使用在大气压力和80°C下工作的连续(光)反应器,在气固状态下研究了(光)催化2-丙醇的脱水反应。PW 12上气相的FTIR光谱记录了先前已吸附了2-丙醇的β-载体复合材料。丙烯和二异丙醚是主要反应产物。对于连续的光辅助运行,反应器也用紫外线照射。在60–120°C范围内确定了2-丙醇催化和光催化脱水的表观活化能。辐照度显着提高了脱水反应速率。在不同的支撑材料之间观察到重要差异。Keggin杂多酸物质在催化和光辅助催化反应中都起着关键作用。实际上,簇的酸度起催化作用,而簇的酸度和PW 12的氧化能力负责光辅助催化反应的反应速率的增加。此外,当将杂多酸负载在TiO 2上时,在固体半导体导带上的光生电子可以说明反应速率的进一步提高。假液相的形成对确定(光)活性起重要作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号