当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering SpyCatcher Variants with Proteolytic Sites for Less‐Trace Ligation
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2018-12-19 , DOI: 10.1002/cjoc.201800475 Xue-Jian Zhang 1 , Xia-Ling Wu 2 , Dong Liu 2 , Xiao-Di Da 2 , Xiao-Wei Wang 2 , Shuguang Yang 1 , Wen-Bin Zhang 2
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2018-12-19 , DOI: 10.1002/cjoc.201800475 Xue-Jian Zhang 1 , Xia-Ling Wu 2 , Dong Liu 2 , Xiao-Di Da 2 , Xiao-Wei Wang 2 , Shuguang Yang 1 , Wen-Bin Zhang 2
Affiliation
|
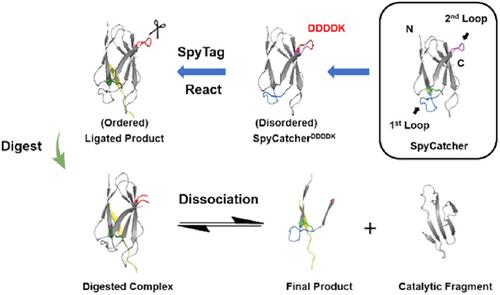
The SpyTag/SpyCatcher reaction is a powerful tool for bioconjugation, but it leaves a complex of considerable size after ligation. To facilitate removal of the catalytic fragment, proteolytic recognition sites (such as DDDDK, AVLQ, and WELQ) were directly engineered into the first or second loop of SpyCatcher at locations after the reactive lysine to give a set of cleavable SpyCatcher variants. Among them, SpyCatcherDDDDK exhibits excellent reactivity with SpyTag and could still be cleaved proteolytically by enterokinase after ligation. Notably, SpyCatcherDDDDK is disordered in solution and forms an ordered complex upon reaction with SpyTag with a second order rate constant of 99.2 ± 0.1 M–1·s–1, which is comparable to, if not faster than, most click reactions. The results demonstrate the high sequence plasticity of SpyCatcher and suggest that covalent bond formation may confer robustness on the folded structure against extensive mutation. These variants add to the expanding toolbox of genetically‐encoded peptide‐protein chemistry with diverse features.
中文翻译:

具有蛋白水解位点的工程SpyCatcher变体,可减少痕量连接
SpyTag / SpyCatcher反应是生物偶联的强大工具,但在连接后会留下相当大的复合物。为了促进催化片段的去除,将蛋白水解识别位点(例如DDDDK,AVLQ和WELQ)直接设计在反应赖氨酸之后的SpyCatcher的第一个或第二个环中,以提供一组可裂解的SpyCatcher变体。其中,SpyCatcher DDDDK与SpyTag具有极好的反应性,并且在连接后仍可被肠激酶进行蛋白水解切割。值得注意的是,SpyCatcher DDDDK在溶液中是无序的,在与SpyTag反应后形成有序的复合物,其二级速率常数为99.2±0.1 M –1 ·s –1,即使不比大多数点击反应快,也可以与之媲美。结果证明了SpyCatcher的高序列可塑性,并表明共价键的形成可能赋予折叠结构抵抗广泛突变的稳健性。这些变体增加了具有多种功能的基因编码肽-蛋白质化学的扩展工具箱。
更新日期:2018-12-19
中文翻译:

具有蛋白水解位点的工程SpyCatcher变体,可减少痕量连接
SpyTag / SpyCatcher反应是生物偶联的强大工具,但在连接后会留下相当大的复合物。为了促进催化片段的去除,将蛋白水解识别位点(例如DDDDK,AVLQ和WELQ)直接设计在反应赖氨酸之后的SpyCatcher的第一个或第二个环中,以提供一组可裂解的SpyCatcher变体。其中,SpyCatcher DDDDK与SpyTag具有极好的反应性,并且在连接后仍可被肠激酶进行蛋白水解切割。值得注意的是,SpyCatcher DDDDK在溶液中是无序的,在与SpyTag反应后形成有序的复合物,其二级速率常数为99.2±0.1 M –1 ·s –1,即使不比大多数点击反应快,也可以与之媲美。结果证明了SpyCatcher的高序列可塑性,并表明共价键的形成可能赋予折叠结构抵抗广泛突变的稳健性。这些变体增加了具有多种功能的基因编码肽-蛋白质化学的扩展工具箱。

































 京公网安备 11010802027423号
京公网安备 11010802027423号