当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reductive Cross-Coupling of Aldehydes and Imines Mediated by Visible Light Photoredox Catalysis
Organic Letters ( IF 4.9 ) Pub Date : 2018-11-28 00:00:00 , DOI: 10.1021/acs.orglett.8b03394 Rui Wang 1 , Mengyue Ma 1 , Xu Gong 1 , Xinyuan Fan 1 , Patrick J. Walsh 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2018-11-28 00:00:00 , DOI: 10.1021/acs.orglett.8b03394 Rui Wang 1 , Mengyue Ma 1 , Xu Gong 1 , Xinyuan Fan 1 , Patrick J. Walsh 1, 2
Affiliation
|
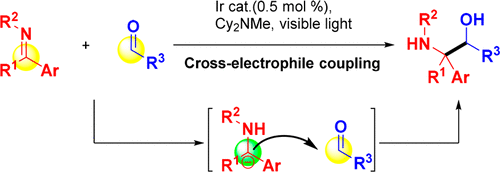
Under photoredox catalysis conditions, the conventional electrophilic reactivity of ketimines is inverted to generate nucleophilic species. As a result, chemoselective cross-electrophile couplings between aldehydes and ketimines are achieved via umpolung reactivity of ketimines to furnish amino alcohols (44 examples with good to excellent yields). To illustrate the utility of the amino alcohol products, 1,2-dihydroindol-3-one-based fluorophores are easily synthesized using the coupling products. Finally, a plausible reaction pathway is discussed.
中文翻译:

可见光光氧化还原催化介导的醛和亚胺的还原性交叉偶联
在光氧化还原催化条件下,酮亚胺的常规亲电反应性被颠倒以产生亲核物质。结果,醛和酮亚胺之间的化学选择性的交叉亲电子偶联是通过酮亚胺与氨基醇的upolung反应来实现的(44个实例,收率高到极好)。为了说明氨基醇产物的效用,使用偶联产物容易地合成基于1,2-二氢吲哚-3-酮的荧光团。最后,讨论了可能的反应途径。
更新日期:2018-11-28
中文翻译:

可见光光氧化还原催化介导的醛和亚胺的还原性交叉偶联
在光氧化还原催化条件下,酮亚胺的常规亲电反应性被颠倒以产生亲核物质。结果,醛和酮亚胺之间的化学选择性的交叉亲电子偶联是通过酮亚胺与氨基醇的upolung反应来实现的(44个实例,收率高到极好)。为了说明氨基醇产物的效用,使用偶联产物容易地合成基于1,2-二氢吲哚-3-酮的荧光团。最后,讨论了可能的反应途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号