当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantitative Determination of Protein-Ligand Affinity by Size Exclusion Chromatography Directly Coupled to High-Resolution Native Mass Spectrometry.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-11-27 00:00:00 , DOI: 10.1021/acs.analchem.8b03829
Chengfeng Ren , Aaron O Bailey 1 , Erica VanderPorten , Angela Oh , Wilson Phung , Melinda M Mulvihill , Seth F Harris , Yichin Liu , Guanghui Han , Wendy Sandoval
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-11-27 00:00:00 , DOI: 10.1021/acs.analchem.8b03829
Chengfeng Ren , Aaron O Bailey 1 , Erica VanderPorten , Angela Oh , Wilson Phung , Melinda M Mulvihill , Seth F Harris , Yichin Liu , Guanghui Han , Wendy Sandoval
Affiliation
|
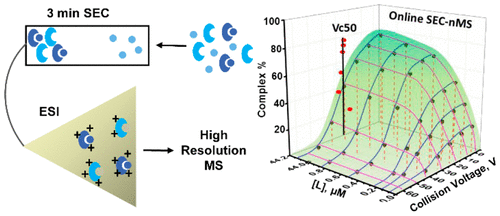
High throughput protein–ligand interaction screening assays employing mass spectrometric detection are widely used in early stage drug discovery. Mass spectrometry-based screening approaches employ a target protein added to a pool of small-molecule compounds, and binding is assessed by measuring ligands denatured from the complexes. Direct analysis of protein–ligand interactions using native mass spectrometry has been demonstrated but is not widely used due to the detection limit on protein size, the requirement of volatile buffers, and the necessity for specialized instrumentation to preserve weak interactions under native conditions. Here we present a robust, quantitative, and automated online size-exclusion chromatography-native mass spectrometry (SEC-nMS) platform for measuring affinities of noncovalent protein–small-molecule interactions on an Orbitrap mass spectrometer. Indoleamine 2,3-dioxygenase 1, a catabolic enzyme, and inhibitory ligands were employed as a demonstration of the method. Efficient separation and elution enabled preservation of protein–ligand complexes and increased throughput. The high sensitivity and intra charge state resolution at high m/z offered by the Exactive Plus EMR Orbitrap allowed for protein ligand affinity quantitation and resolved individual compounds close in mass. Vc50 values determined via collision-induced dissociation experiments enabled the evaluation of complex stability in the gas phase and were found to be independent of the extent of complex formation. For the first time, Vc50 determinations were achieved on an inline SEC-nMS platform. Systematic comparison of our method with optimized chip-based nanoelectrospray infusion served as a reference for ligand screening and affinity quantitation and further revealed the advantages of SEC-MS.
中文翻译:

通过直接与高分辨率原生质谱联用的尺寸排阻色谱法定量测定蛋白质-配体亲和力。
采用质谱检测的高通量蛋白质-配体相互作用筛选分析广泛用于早期药物发现。基于质谱的筛选方法采用添加到小分子化合物库中的目标蛋白,并通过测量从复合物变性的配体来评估结合。使用天然质谱法直接分析蛋白质 - 配体相互作用已得到证实,但由于蛋白质大小的检测限制、挥发性缓冲液的要求以及专用仪器在天然条件下保持弱相互作用的必要性,因此并未广泛使用。在这里,我们提出了一个稳健的、定量的、和自动在线尺寸排阻色谱-原生质谱 (SEC-nMS) 平台,用于在 Orbitrap 质谱仪上测量非共价蛋白质-小分子相互作用的亲和力。吲哚胺 2,3-双加氧酶 1、一种分解代谢酶和抑制性配体被用作该方法的演示。有效的分离和洗脱能够保存蛋白质-配体复合物并提高通量。高灵敏度和内部电荷态分辨率Exactive Plus EMR Orbitrap 提供的m / z允许进行蛋白质配体亲和定量并解析质量接近的单个化合物。通过碰撞诱导解离实验确定的Vc 50值能够评估气相中的复合物稳定性,并且被发现与复合物形成的程度无关。首次在在线 SEC-nMS 平台上实现了Vc 50 的测定。我们的方法与优化的基于芯片的纳米电喷雾输注的系统比较作为配体筛选和亲和定量的参考,并进一步揭示了 SEC-MS 的优势。
更新日期:2018-11-27
中文翻译:

通过直接与高分辨率原生质谱联用的尺寸排阻色谱法定量测定蛋白质-配体亲和力。
采用质谱检测的高通量蛋白质-配体相互作用筛选分析广泛用于早期药物发现。基于质谱的筛选方法采用添加到小分子化合物库中的目标蛋白,并通过测量从复合物变性的配体来评估结合。使用天然质谱法直接分析蛋白质 - 配体相互作用已得到证实,但由于蛋白质大小的检测限制、挥发性缓冲液的要求以及专用仪器在天然条件下保持弱相互作用的必要性,因此并未广泛使用。在这里,我们提出了一个稳健的、定量的、和自动在线尺寸排阻色谱-原生质谱 (SEC-nMS) 平台,用于在 Orbitrap 质谱仪上测量非共价蛋白质-小分子相互作用的亲和力。吲哚胺 2,3-双加氧酶 1、一种分解代谢酶和抑制性配体被用作该方法的演示。有效的分离和洗脱能够保存蛋白质-配体复合物并提高通量。高灵敏度和内部电荷态分辨率Exactive Plus EMR Orbitrap 提供的m / z允许进行蛋白质配体亲和定量并解析质量接近的单个化合物。通过碰撞诱导解离实验确定的Vc 50值能够评估气相中的复合物稳定性,并且被发现与复合物形成的程度无关。首次在在线 SEC-nMS 平台上实现了Vc 50 的测定。我们的方法与优化的基于芯片的纳米电喷雾输注的系统比较作为配体筛选和亲和定量的参考,并进一步揭示了 SEC-MS 的优势。







































 京公网安备 11010802027423号
京公网安备 11010802027423号