当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Differentiation between enamines and tautomerizable imines in the oxidation reaction with TEMPO.
Nature Communications ( IF 14.7 ) Pub Date : 2018-11-27 , DOI: 10.1038/s41467-018-07534-x Xiaoming Jie , Yaping Shang , Zhe-Ning Chen , Xiaofeng Zhang , Wei Zhuang , Weiping Su
Nature Communications ( IF 14.7 ) Pub Date : 2018-11-27 , DOI: 10.1038/s41467-018-07534-x Xiaoming Jie , Yaping Shang , Zhe-Ning Chen , Xiaofeng Zhang , Wei Zhuang , Weiping Su
|
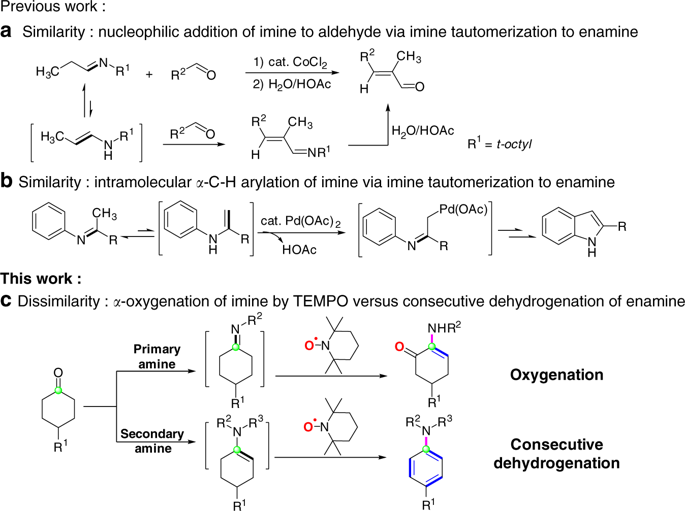
Enamine and imine represent two of the most common reaction intermediates in syntheses, and the imine intermediates containing α-hydrogen often exhibit the similar reactivity to enamines due to their rapid tautomerization to enamine tautomers. Herein, we report that the minor structural difference between the enamine and the enamine tautomer derived from imine tautomerization results in the different chemo- and regioselectivity in the reaction of cyclohexanones, amines and TEMPO: the reaction of primary amines furnishes the formal oxygen 1,2-migration product, α-amino-enones, while the reaction of secondary amines under similar conditions generates exclusively arylamines via consecutive dehydrogenation on the cyclohexyl rings. The 18O-labeling experiment for α-amino-enone formation revealed that TEMPO served as oxygen transfer reagent. Experimental and computational studies of reaction mechanisms revealed that the difference in chemo- and regioselectivity could be ascribed to the flexible imine-enamine tautomerization of the imine intermediate containing an α-hydrogen.
中文翻译:

TEMPO氧化反应中烯胺和互变异构亚胺的区别。
烯胺和亚胺是合成中最常见的两种反应中间体,而含α-氢的亚胺中间体由于与烯胺互变异构体的快速互变异构作用,经常表现出与烯胺相似的反应性。在此,我们报道烯胺和亚胺互变异构衍生的烯胺互变异构体之间的微小结构差异导致环己酮,胺和TEMPO反应的化学选择性和区域选择性不同:伯胺的反应提供了形式氧1,2 -迁移产物α-氨基烯酮,而仲胺在相似条件下的反应则通过在环己基环上连续脱氢而仅生成芳基胺。在18O标记形成α-氨基烯酮的实验表明,TEMPO可作为氧转移试剂。反应机理的实验和计算研究表明,化学选择性和区域选择性的差异可归因于含α-氢的亚胺中间体的柔性亚胺-烯胺互变异构。
更新日期:2018-11-28
中文翻译:

TEMPO氧化反应中烯胺和互变异构亚胺的区别。
烯胺和亚胺是合成中最常见的两种反应中间体,而含α-氢的亚胺中间体由于与烯胺互变异构体的快速互变异构作用,经常表现出与烯胺相似的反应性。在此,我们报道烯胺和亚胺互变异构衍生的烯胺互变异构体之间的微小结构差异导致环己酮,胺和TEMPO反应的化学选择性和区域选择性不同:伯胺的反应提供了形式氧1,2 -迁移产物α-氨基烯酮,而仲胺在相似条件下的反应则通过在环己基环上连续脱氢而仅生成芳基胺。在18O标记形成α-氨基烯酮的实验表明,TEMPO可作为氧转移试剂。反应机理的实验和计算研究表明,化学选择性和区域选择性的差异可归因于含α-氢的亚胺中间体的柔性亚胺-烯胺互变异构。































 京公网安备 11010802027423号
京公网安备 11010802027423号