当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of (±)-Serralongamine A and the Revised Structure of Huperzine N
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2016-03-04 00:00:00 , DOI: 10.1021/acs.joc.6b00025 Gisela V. Saborit 1 , Caroline Bosch 1 , Teodor Parella 2 , Ben Bradshaw 1 , Josep Bonjoch 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2016-03-04 00:00:00 , DOI: 10.1021/acs.joc.6b00025 Gisela V. Saborit 1 , Caroline Bosch 1 , Teodor Parella 2 , Ben Bradshaw 1 , Josep Bonjoch 1
Affiliation
|
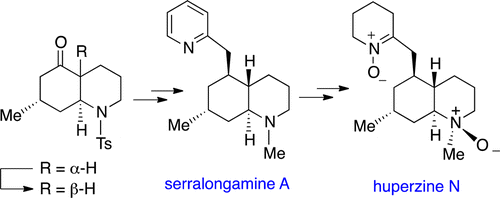
A revised structure for the Lycopodium alkaloid huperzine N is proposed and confirmed by synthesis. The key synthetic steps involve an epimerization of a cis-5-oxodecahydroquinoline to the corresponding trans isomer and a coupling, followed by a diastereoselective hydrogenation using Wilkinson’s catalyst to incorporate the pyridylmethyl moiety. This route allowed the alkaloid serralongamine A to be synthesized for the first time, and two additional steps led to the revised structure of huperzine N, both products bearing an unusual decahydroquinoline stereostructure.
中文翻译:

(±)-Serralongamine A的合成和石杉碱N的修饰结构
提出了经修饰的石蒜碱生物碱石杉碱N的结构,并通过合成证实。关键的合成步骤包括将顺式-5-氧代十二氢喹啉差向异构化为相应的反式异构体并进行偶联,然后使用威尔金森氏催化剂进行非对映选择性氢化以引入吡啶基甲基部分。该途径使生物碱塞拉隆明A首次得以合成,另外两个步骤导致石杉碱N的结构发生了改变,两种产物均具有不寻常的十氢喹啉立体结构。
更新日期:2016-03-04
中文翻译:

(±)-Serralongamine A的合成和石杉碱N的修饰结构
提出了经修饰的石蒜碱生物碱石杉碱N的结构,并通过合成证实。关键的合成步骤包括将顺式-5-氧代十二氢喹啉差向异构化为相应的反式异构体并进行偶联,然后使用威尔金森氏催化剂进行非对映选择性氢化以引入吡啶基甲基部分。该途径使生物碱塞拉隆明A首次得以合成,另外两个步骤导致石杉碱N的结构发生了改变,两种产物均具有不寻常的十氢喹啉立体结构。































 京公网安备 11010802027423号
京公网安备 11010802027423号