当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Active Platinum Catalysts for Nitrile and Cyanohydrin Hydration: Catalyst Design and Ligand Screening via High-Throughput Techniques
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-11-27 , DOI: 10.1021/jacs.8b11667
Xiangyou Xing 1, 2 , Chen Xu 1, 2 , Bo Chen 1 , Chengcheng Li 1 , Scott C. Virgil 2 , Robert H. Grubbs 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-11-27 , DOI: 10.1021/jacs.8b11667
Xiangyou Xing 1, 2 , Chen Xu 1, 2 , Bo Chen 1 , Chengcheng Li 1 , Scott C. Virgil 2 , Robert H. Grubbs 2
Affiliation
|
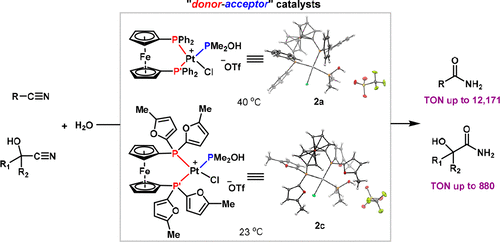
Nitrile hydration provides access to amides that are indispensable to researchers in chemical and pharmaceutical industries. Prohibiting the use of this venerable reaction, however, are (1) the dearth of biphasic catalysts that can effectively hydrate nitriles at ambient temperatures with high turnover numbers and (2) the unsolved challenge of hydrating cyanohydrins. Herein, we report the design of new " donor-acceptor"-type platinum catalysts by precisely arranging electron-rich and electron-deficient ligands trans to one other, thereby enhancing both the nucleophilicity of the hydroxyl group and the electrophilicity of the nitrile group. Leveraging a high-throughput, automated workflow and evaluating a library of bidentate ligands, we have discovered that commercially available, inexpensive DPPF [1,1'-ferrocenendiyl-bis(diphenylphosphine)] provides superior reactivity. The corresponding " donor-acceptor"-type catalyst 2a is readily prepared from (DPPF)PtCl2, PMe2OH, and AgOTf. The enhanced activity of 2a permits the hydration of a wide range of nitriles and cyanohydrins to proceed at 40 °C with excellent turnover numbers. Rational reevaluation of the ligand structure has led to the discovery of modified catalyst 2c, harboring the more electron-rich 1,1'-bis[bis(5-methyl-2-furanyl)phosphino] ferrocene ligand, which demonstrates the highest activity toward hydration of nitriles and cyanohydrins at room temperature. Finally, the correlation between the electron-donating ability of the phosphine ligands with catalyst efficiencies of 2a, 2c, 2d, and 2e in the hydration of nitrile 7 are examined, and the results support the " donor-acceptor" hypothesis.
中文翻译:

用于腈和氰醇水合的高活性铂催化剂:通过高通量技术进行催化剂设计和配体筛选
腈水合为化学和制药行业的研究人员提供了必不可少的酰胺。然而,禁止使用这种古老的反应是 (1) 缺乏能够在环境温度下以高转换数有效水合腈类的双相催化剂,以及 (2) 水合氰醇的未解决挑战。在此,我们报告了通过精确地将富电子和缺电子配体相互反式排列,从而增强羟基的亲核性和腈基的亲电性来设计新型“供体-受体”型铂催化剂。利用高通量、自动化的工作流程并评估双齿配体库,我们发现了市售的廉价 DPPF [1,1' -ferrocenendiyl-bis(diphenylphosphine)] 提供优异的反应性。相应的“供体-受体”型催化剂2a很容易由(DPPF)PtCl2、PMe2OH和AgOTf制备。2a 的增强活性允许各种腈和氰醇在 40 °C 下以优异的转化率进行水合。对配体结构的合理重新评估导致发现了改性催化剂 2c,它含有更多富电子的 1,1'-双[双(5-甲基-2-呋喃基)膦基]二茂铁配体,其对腈和氰醇在室温下水合。最后,考察了膦配体的给电子能力与 2a、2c、2d 和 2e 在腈 7 水合中的催化效率之间的相关性,结果支持“
更新日期:2018-11-27
中文翻译:

用于腈和氰醇水合的高活性铂催化剂:通过高通量技术进行催化剂设计和配体筛选
腈水合为化学和制药行业的研究人员提供了必不可少的酰胺。然而,禁止使用这种古老的反应是 (1) 缺乏能够在环境温度下以高转换数有效水合腈类的双相催化剂,以及 (2) 水合氰醇的未解决挑战。在此,我们报告了通过精确地将富电子和缺电子配体相互反式排列,从而增强羟基的亲核性和腈基的亲电性来设计新型“供体-受体”型铂催化剂。利用高通量、自动化的工作流程并评估双齿配体库,我们发现了市售的廉价 DPPF [1,1' -ferrocenendiyl-bis(diphenylphosphine)] 提供优异的反应性。相应的“供体-受体”型催化剂2a很容易由(DPPF)PtCl2、PMe2OH和AgOTf制备。2a 的增强活性允许各种腈和氰醇在 40 °C 下以优异的转化率进行水合。对配体结构的合理重新评估导致发现了改性催化剂 2c,它含有更多富电子的 1,1'-双[双(5-甲基-2-呋喃基)膦基]二茂铁配体,其对腈和氰醇在室温下水合。最后,考察了膦配体的给电子能力与 2a、2c、2d 和 2e 在腈 7 水合中的催化效率之间的相关性,结果支持“







































 京公网安备 11010802027423号
京公网安备 11010802027423号