当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation, Crystal Structures, and Oral Bioavailability of Two Cocrystals of Emodin with Berberine Chloride
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2018-11-26 00:00:00 , DOI: 10.1021/acs.cgd.8b01257 Yanping Deng 1 , Yanjie Zhang 2 , Yali Huang 2 , Mei Zhang 2 , Benyong Lou 2
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2018-11-26 00:00:00 , DOI: 10.1021/acs.cgd.8b01257 Yanping Deng 1 , Yanjie Zhang 2 , Yali Huang 2 , Mei Zhang 2 , Benyong Lou 2
Affiliation
|
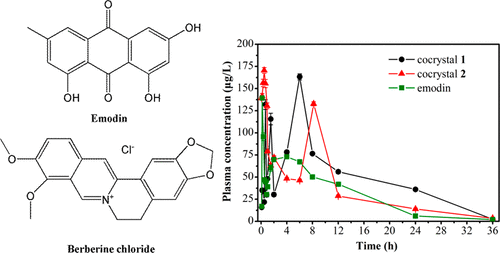
Two cocrystals of emodin (EM) with berberine chloride (BER), EM-BER (1) and 2EM-BER-EtOH (2), were prepared and characterized. There exist reliable O-H···Cl– hydrogen-bonding interactions between the 2-hydroxyl group of emodin and chloride anion. Various π–π interactions dominate the packing structures of 1 and 2. In 1, emodin molecules stack into a supramolecular layer through π–π interactions while π–π interactions between berberine cations also result in a similar layer. In 2, berberine cation simultaneously interacts with two different emodin through π–π interactions to give rise to a 1D chainlike array. Cocrystals 1 and 2 present a low moisture adsorption curve in the range of 0–95% relative humidity values at 25 °C. The sustained release of berberine chloride in pure water could be achieved after forming cocrystals 1 and 2. Emodin in the form of cocrystals 1 and 2 has a higher Cmax and AUC compared with pure emodin.
中文翻译:

大黄素与氯化小Ber碱的两个共晶体的制备,晶体结构和口服生物利用度
制备并表征了大黄素(EM)与小碱氯化物(BER)的两个共晶体EM-BER(1)和2EM-BER-EtOH(2)。大黄素的2-羟基与氯离子之间存在可靠的OH···Cl –氢键相互作用。各种π-π相互作用主导1和2的堆积结构。在图1中,大黄素分子通过π–π相互作用堆积成超分子层,而小ber碱阳离子之间的π–π相互作用也产生了类似的层。在2中,小ber碱阳离子同时通过π-π相互作用与两种不同的大黄素相互作用,从而产生一维链状阵列。共晶1和图2显示了在25°C时相对湿度值在0–95%范围内的低水分吸附曲线。形成共晶1和2后,可以实现在纯水中持续释放小碱氯化物。与纯大黄素相比,共晶1和2形式的大黄素具有更高的C max和AUC。
更新日期:2018-11-26
中文翻译:

大黄素与氯化小Ber碱的两个共晶体的制备,晶体结构和口服生物利用度
制备并表征了大黄素(EM)与小碱氯化物(BER)的两个共晶体EM-BER(1)和2EM-BER-EtOH(2)。大黄素的2-羟基与氯离子之间存在可靠的OH···Cl –氢键相互作用。各种π-π相互作用主导1和2的堆积结构。在图1中,大黄素分子通过π–π相互作用堆积成超分子层,而小ber碱阳离子之间的π–π相互作用也产生了类似的层。在2中,小ber碱阳离子同时通过π-π相互作用与两种不同的大黄素相互作用,从而产生一维链状阵列。共晶1和图2显示了在25°C时相对湿度值在0–95%范围内的低水分吸附曲线。形成共晶1和2后,可以实现在纯水中持续释放小碱氯化物。与纯大黄素相比,共晶1和2形式的大黄素具有更高的C max和AUC。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号