当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rare-earth ion exchanged Cu-SSZ-13 zeolite from organotemplate-free synthesis with enhanced hydrothermal stability in NH3-SCR of NOx†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-11-26 00:00:00 , DOI: 10.1039/c8cy02033g Zhenchao Zhao 1, 2, 3, 4, 5 , Rui Yu 1, 2, 3, 4, 5 , Chuan Shi 1, 2, 3, 4, 5 , Hermann Gies 6, 7, 8, 9 , Feng-Shou Xiao 5, 10, 11, 12 , Dirk De Vos 13, 14, 15, 16 , Toshiyuki Yokoi 17, 18, 19, 20 , Xinhe Bao 5, 21, 22, 23 , Ute Kolb 9, 24, 25, 26 , Robert McGuire 9, 27, 28, 29 , Andrei-Nicolae Parvulescu 9, 27, 28, 29 , Stefan Maurer 9, 27, 28, 29 , Ulrich Müller 9, 27, 28, 29 , Weiping Zhang 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-11-26 00:00:00 , DOI: 10.1039/c8cy02033g Zhenchao Zhao 1, 2, 3, 4, 5 , Rui Yu 1, 2, 3, 4, 5 , Chuan Shi 1, 2, 3, 4, 5 , Hermann Gies 6, 7, 8, 9 , Feng-Shou Xiao 5, 10, 11, 12 , Dirk De Vos 13, 14, 15, 16 , Toshiyuki Yokoi 17, 18, 19, 20 , Xinhe Bao 5, 21, 22, 23 , Ute Kolb 9, 24, 25, 26 , Robert McGuire 9, 27, 28, 29 , Andrei-Nicolae Parvulescu 9, 27, 28, 29 , Stefan Maurer 9, 27, 28, 29 , Ulrich Müller 9, 27, 28, 29 , Weiping Zhang 1, 2, 3, 4, 5
Affiliation
|
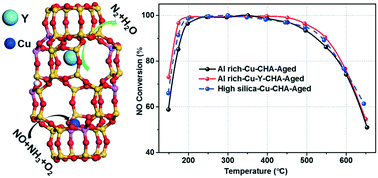
The relatively low hydrothermal stability of Al-rich Cu-SSZ-13 catalysts hinders their practical application in ammonia selective catalytic reduction (NH3-SCR) reaction. Rare-earth ions were introduced into the Al-rich SSZ-13 zeolite using an organotemplate-free synthesis prior to the exchange of Cu2+ ions. Among the rare-earth ions tested (Ce, La, Sm, Y, Yb), Y shows significant enhancement of the hydrothermal stability and NH3-SCR activities after severe hydrothermal aging at 800 °C for 16 h when compared with Cu-SSZ-13 without Y. Cu–Y-SSZ-13 catalysts with various amounts of Y were prepared, and it is found that with increasing Y content, the low temperature NO conversions can be improved even after hydrothermal aging. SEM-EDX analysis together with two-dimensional multiple quantum magic-angle-spinning nuclear magnetic resonance (23Na MQ MAS NMR) confirms that the Y ions are successfully incorporated into the ion-exchange sites of the SSZ-13 zeolite. Results from 27Al MAS, 29Si MAS NMR, temperature-programmed desorption of ammonia (NH3-TPD) and quantitative 1H MAS NMR indicate that Y can stabilize the framework Al and also preserve the Brønsted acid sites in the Al-rich SSZ-13 zeolite. The hydrogen temperature programmed reduction (H2-TPR), ultraviolet-visible-near infrared spectroscopy (UV-vis-NIR) and diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) of nitric oxide (NO) or NH3 adsorption demonstrate that introduction of Y ions causes Cu2+ ions to preferentially occupy the 6-MR, which has high hydrothermal stability. However, too much of Y may lead to activity loss at both low and high temperatures. The optimized Al-rich Cu–Y-SSZ-13 with 2.8 wt% of copper (Cu) and 1.3 wt% of Y displays almost the same deNOx activities as the conventional organotemplated high silica Cu-SSZ-13 catalyst in a wide reaction temperature window of 150–650 °C after severe hydrothermal treatment. Rare-earth ions could be an effective additive for Cu-SSZ-13 catalysts to further improve their hydrothermal stability for practical applications.
中文翻译:

无有机模板合成中的稀土离子交换Cu-SSZ-13沸石,在NO x †的NH 3 -SCR中具有增强的水热稳定性
富铝的Cu-SSZ-13催化剂相对较低的水热稳定性阻碍了其在氨选择性催化还原(NH 3 -SCR)反应中的实际应用。在交换Cu 2+离子之前,使用无有机模板的合成方法将稀土离子引入到富含Al的SSZ-13沸石中。在测试的稀土离子(Ce,La,Sm,Y,Yb)中,Y显示出水热稳定性和NH 3的显着增强与不含Y的Cu-SSZ-13相比,在800°C严重水热老化16 h后的-SCR活性。制备了各种Y量的Cu–Y-SSZ-13催化剂,发现随着Y含量的增加,即使在水热老化之后,也可以改善低温NO的转化率。SEM-EDX分析与二维多重量子魔术角自旋核磁共振(23 Na MQ MAS NMR)一起证实了Y离子已成功地掺入SSZ-13沸石的离子交换位中。结果来自27 Al MAS,29 Si MAS NMR,程序升温的氨解吸(NH 3 -TPD)和定量1H MAS NMR表明Y可以稳定框架Al,并且还可以在富含Al的SSZ-13沸石中保留布朗斯台德酸位。一氧化氮(NO)或NH 3吸附的氢程序升温还原(H 2 -TPR),紫外可见-近红外光谱(UV-vis-NIR)和漫反射红外傅里叶变换光谱(DRIFTS)表明引入了Y离子使Cu 2+离子优先占据6-MR,具有较高的水热稳定性。但是,过多的Y可能会在低温和高温下导致活性下降。经过优化的富含Al的Cu–Y-SSZ-13具有2.8 wt%的铜(Cu)和1.3 wt%的Y,其脱氮x几乎相同在经过严格的水热处理后,可在150-650°C的宽反应温度范围内发挥常规有机模板化高硅铜Cu-SSZ-13催化剂的活性。稀土离子可能是Cu-SSZ-13催化剂的有效添加剂,可以进一步提高其水热稳定性,以供实际应用。
更新日期:2018-11-26
中文翻译:

无有机模板合成中的稀土离子交换Cu-SSZ-13沸石,在NO x †的NH 3 -SCR中具有增强的水热稳定性
富铝的Cu-SSZ-13催化剂相对较低的水热稳定性阻碍了其在氨选择性催化还原(NH 3 -SCR)反应中的实际应用。在交换Cu 2+离子之前,使用无有机模板的合成方法将稀土离子引入到富含Al的SSZ-13沸石中。在测试的稀土离子(Ce,La,Sm,Y,Yb)中,Y显示出水热稳定性和NH 3的显着增强与不含Y的Cu-SSZ-13相比,在800°C严重水热老化16 h后的-SCR活性。制备了各种Y量的Cu–Y-SSZ-13催化剂,发现随着Y含量的增加,即使在水热老化之后,也可以改善低温NO的转化率。SEM-EDX分析与二维多重量子魔术角自旋核磁共振(23 Na MQ MAS NMR)一起证实了Y离子已成功地掺入SSZ-13沸石的离子交换位中。结果来自27 Al MAS,29 Si MAS NMR,程序升温的氨解吸(NH 3 -TPD)和定量1H MAS NMR表明Y可以稳定框架Al,并且还可以在富含Al的SSZ-13沸石中保留布朗斯台德酸位。一氧化氮(NO)或NH 3吸附的氢程序升温还原(H 2 -TPR),紫外可见-近红外光谱(UV-vis-NIR)和漫反射红外傅里叶变换光谱(DRIFTS)表明引入了Y离子使Cu 2+离子优先占据6-MR,具有较高的水热稳定性。但是,过多的Y可能会在低温和高温下导致活性下降。经过优化的富含Al的Cu–Y-SSZ-13具有2.8 wt%的铜(Cu)和1.3 wt%的Y,其脱氮x几乎相同在经过严格的水热处理后,可在150-650°C的宽反应温度范围内发挥常规有机模板化高硅铜Cu-SSZ-13催化剂的活性。稀土离子可能是Cu-SSZ-13催化剂的有效添加剂,可以进一步提高其水热稳定性,以供实际应用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号