Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Epigenetic Heterogeneity in Human Colorectal Tumors Reveals Preferential Conservation And Evidence of Immune Surveillance.
Scientific Reports ( IF 3.8 ) Pub Date : 2018-11-23 , DOI: 10.1038/s41598-018-35621-y Marc D Ryser 1, 2, 3 , Ming Yu 4 , William Grady 4, 5 , Kimberly Siegmund 6 , Darryl Shibata 7
Scientific Reports ( IF 3.8 ) Pub Date : 2018-11-23 , DOI: 10.1038/s41598-018-35621-y Marc D Ryser 1, 2, 3 , Ming Yu 4 , William Grady 4, 5 , Kimberly Siegmund 6 , Darryl Shibata 7
Affiliation
|
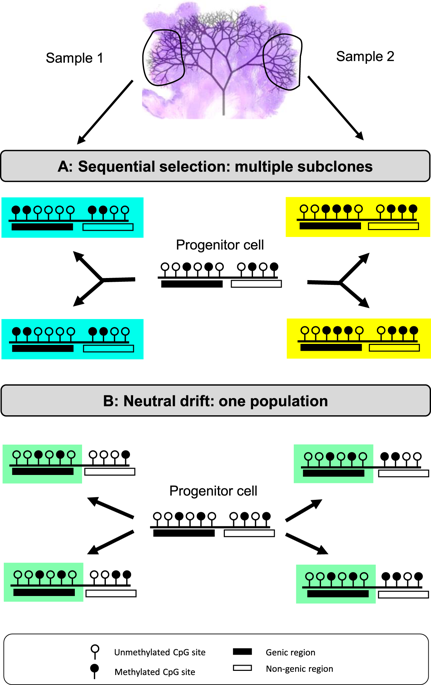
Genomic intratumoral heterogeneity (ITH) is common in cancers, but the extent of phenotypic ITH is uncertain because most subclonal mutations are passengers. Since tumor phenotypes are largely driven by epigenetics, methylomic analyses can provide insights into phenotypic ITH. Following this principle, we determined the extent of epigenetic ITH in 16 human colorectal tumors by comparing the methylomes from spatially separated regions in each tumor. Methylomes from opposite tumor sides were similar (Pearson correlation >0.95) with little evidence of ITH or stepwise selection during growth, suggesting that the epigenome of a sampled tumor largely reflects that of its founder cell. Epigenetic conservation was functional, with higher conservation at promoters and expressed genes compared to non-coding regions. Despite epigenomic conservation, RNA expression varied between individual tumor glands, indicating continued adaption during growth. Because many promoters and enhancers were unmethylated, continued adaptation may be due to phenotypic plasticity. Gene enrichment analyses identified that interferon signaling and antigen-processing and presenting pathways were strongly conserved during tumor growth, suggesting a mechanism for immune evasion. In summary, our findings suggest that epigenomes are preferentially conserved during tumor growth and that early tumor cells are poised for rapid growth, phenotypic adaptation, and immune evasion.
中文翻译:

人类大肠肿瘤的表观遗传异质性显示了保守性保守和免疫监测的证据。
基因组肿瘤内异质性(ITH)在癌症中很常见,但表型ITH的程度尚不确定,因为大多数亚克隆突变都是客体。由于肿瘤表型在很大程度上由表观遗传学驱动,因此甲基组学分析可以提供对表型ITH的洞察力。遵循这一原理,我们通过比较每个肿瘤在空间上分开的区域中的甲基化组,确定了16种人类大肠肿瘤中表观遗传ITH的程度。来自相反肿瘤侧的甲基化组相似(Pearson相关性> 0.95),几乎没有生长过程中ITH或逐步选择的证据,表明所采样肿瘤的表观组在很大程度上反映了其始建细胞的表观。表观遗传保守是功能性的,与非编码区相比,在启动子和表达的基因上具有更高的保守性。尽管表观基因组保守,RNA的表达在各个肿瘤腺体之间不同,表明在生长过程中持续适应。由于许多启动子和增强子未甲基化,因此持续的适应性可能归因于表型可塑性。基因富集分析表明,在肿瘤生长过程中,干扰素的信号传导,抗原加工和呈递途径被高度保守,这提示了免疫逃逸的机制。总而言之,我们的发现表明表观基因组在肿瘤生长过程中是优先保守的,早期肿瘤细胞已准备好快速生长,表型适应和免疫逃避。基因富集分析表明,在肿瘤生长过程中,干扰素的信号传导,抗原加工和呈递途径被高度保守,这提示了免疫逃逸的机制。总而言之,我们的发现表明表观基因组在肿瘤生长过程中是优先保守的,早期肿瘤细胞已准备好快速生长,表型适应和免疫逃避。基因富集分析表明,在肿瘤生长过程中,干扰素的信号传导,抗原加工和呈递途径被高度保守,这提示了免疫逃逸的机制。总而言之,我们的发现表明表观基因组在肿瘤生长过程中被优先保守,并且早期肿瘤细胞已准备好快速生长,表型适应和免疫逃避。
更新日期:2018-11-26
中文翻译:

人类大肠肿瘤的表观遗传异质性显示了保守性保守和免疫监测的证据。
基因组肿瘤内异质性(ITH)在癌症中很常见,但表型ITH的程度尚不确定,因为大多数亚克隆突变都是客体。由于肿瘤表型在很大程度上由表观遗传学驱动,因此甲基组学分析可以提供对表型ITH的洞察力。遵循这一原理,我们通过比较每个肿瘤在空间上分开的区域中的甲基化组,确定了16种人类大肠肿瘤中表观遗传ITH的程度。来自相反肿瘤侧的甲基化组相似(Pearson相关性> 0.95),几乎没有生长过程中ITH或逐步选择的证据,表明所采样肿瘤的表观组在很大程度上反映了其始建细胞的表观。表观遗传保守是功能性的,与非编码区相比,在启动子和表达的基因上具有更高的保守性。尽管表观基因组保守,RNA的表达在各个肿瘤腺体之间不同,表明在生长过程中持续适应。由于许多启动子和增强子未甲基化,因此持续的适应性可能归因于表型可塑性。基因富集分析表明,在肿瘤生长过程中,干扰素的信号传导,抗原加工和呈递途径被高度保守,这提示了免疫逃逸的机制。总而言之,我们的发现表明表观基因组在肿瘤生长过程中是优先保守的,早期肿瘤细胞已准备好快速生长,表型适应和免疫逃避。基因富集分析表明,在肿瘤生长过程中,干扰素的信号传导,抗原加工和呈递途径被高度保守,这提示了免疫逃逸的机制。总而言之,我们的发现表明表观基因组在肿瘤生长过程中是优先保守的,早期肿瘤细胞已准备好快速生长,表型适应和免疫逃避。基因富集分析表明,在肿瘤生长过程中,干扰素的信号传导,抗原加工和呈递途径被高度保守,这提示了免疫逃逸的机制。总而言之,我们的发现表明表观基因组在肿瘤生长过程中被优先保守,并且早期肿瘤细胞已准备好快速生长,表型适应和免疫逃避。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号