当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactions of an Osmium(IV)-Hydroxo Complex with Amino-Boranes: Formation of Boroxide Derivatives
Organometallics ( IF 2.5 ) Pub Date : 2018-11-21 , DOI: 10.1021/acs.organomet.8b00727 Antonio Antiñolo 1 , Miguel A. Esteruelas 2 , Cristina García-Yebra 2 , Jaime Martín 2 , Enrique Oñate 2 , Alberto Ramos 1
Organometallics ( IF 2.5 ) Pub Date : 2018-11-21 , DOI: 10.1021/acs.organomet.8b00727 Antonio Antiñolo 1 , Miguel A. Esteruelas 2 , Cristina García-Yebra 2 , Jaime Martín 2 , Enrique Oñate 2 , Alberto Ramos 1
Affiliation
|
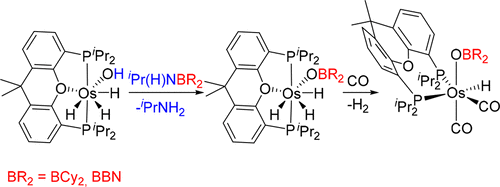
The discovery of a reaction which allows preparation of boroxide complexes of platinum group metals and study of their behavior under CO atmosphere is described. The trihydride-osmium(IV)-hydroxo complex OsH3(OH){κ3-P,O,P-[xant(PiPr2)2]} (1, xant(PiPr2)2 = 4,5-bis(diisopropylphosphino)xanthene) reacts with the amino-boranes iPr(H)NBCy2 and iPr(H)NBBN to give the osmium(IV)-boroxide derivatives OsH3(OBR2){κ3-P,O,P-[xant(PiPr2)2]} (BR2 = BCy2 (2), BBN (3); BBN = 9-borabicyclo[3.5.1]nonane) and iPrNH2 as a consequence of the addition of the O–H bond of the hydroxo ligand of 1 to the B–N bond of the amino-boranes. At room temperature under CO atmosphere, complexes 2 and 3 eliminate H2 to afford the osmium(II)–boroxide compounds OsH(OBR2)(CO)2{κ2-P,P-[xant(PiPr2)2]} (BR2 = BCy2 (4), BBN (5)) bearing a κ2-P,P-coordinated ether-diphosphine. The subsequent reductive elimination of the borinic acids R2BOH needs heating and a long duration and leads to the tricarbonyl-osmium(0) derivative Os(CO)3{κ2-P,P-[xant(PiPr2)2]} (6) with the phosphorus atoms of the diphosphine lying in the equatorial plane of a pentagonal bypyramid of donor atoms around the metal center. In contrast to 2 and 3, under CO atmosphere, precursor 1 eliminates water to initially give the trans-dihydride OsH2(CO){κ3-P,O,P-[xant(PiPr2)2]} (7), which subsequently evolves to the cis-dihydride-cis-dicarbonyl derivative OsH2(CO)2{κ2-P,P-[xant(PiPr2)2]} (8) and finally into the tricarbonyl 6.
中文翻译:

IV(IV)-羟基配合物与氨基硼烷的反应:硼氧化物衍生物的形成
描述了允许制备铂族金属的硼氧化物络合物的反应的发现并研究了它们在CO气氛下的行为。所述三氢化-锇(IV)-hydroxo复杂OSH 3(OH){κ 3 - P,ö,P - 〔xant(P我镨2)2 ]}(1,xant(P我镨2)2 = 4, 5-双(二异丙基膦基)氧杂蒽并与氨基硼烷i Pr(H)NBCy 2和i Pr(H)NBBN反应生成give(IV)-硼氧化物衍生物OsH 3(OBR 2){κ 3 - P,ö,P - 〔xant(P我镨2)2 ]}(BR 2 = BCY 2(2),BBN(3); BBN = 9-硼杂双环[3.5.1]壬烷)和i PrNH 2是由于1的羟基配体的O–H键加到氨基硼烷的B–N键上的结果。在室温和CO气氛下,配合物2和3消除H 2,得到bor(II)-硼氧化物OsH(OBR 2)(CO)2 {κ2 - P,P - 〔xant(P我镨2)2 ]}(BR 2 = BCY 2(4),BBN(5))轴承κ 2 - P,P配位的醚-二膦。的二取代硼酸随后的还原消除- [R 2只BOH需要加热和长持续时间,并导致三羰基锇(0)衍生物OS(CO)3 {κ 2 - P,P - 〔xant(P我镨2)2 ]}(6),其中二膦的磷原子位于金属中心周围的供体原子的五角形金字塔的赤道平面内。在对比2和3,CO气氛下,前体1个消除水最初得到反式-dihydride OSH 2(CO){κ 3 - P,ö,P - 〔xant(P我镨2)2 ]}(7),其随后演变到顺-dihydride-顺二羰基衍生物OSH 2(CO)2 {κ 2- P,P - 〔xant(P我镨2)2 ]}(8),最后进入三羰基6。
更新日期:2018-11-24
中文翻译:

IV(IV)-羟基配合物与氨基硼烷的反应:硼氧化物衍生物的形成
描述了允许制备铂族金属的硼氧化物络合物的反应的发现并研究了它们在CO气氛下的行为。所述三氢化-锇(IV)-hydroxo复杂OSH 3(OH){κ 3 - P,ö,P - 〔xant(P我镨2)2 ]}(1,xant(P我镨2)2 = 4, 5-双(二异丙基膦基)氧杂蒽并与氨基硼烷i Pr(H)NBCy 2和i Pr(H)NBBN反应生成give(IV)-硼氧化物衍生物OsH 3(OBR 2){κ 3 - P,ö,P - 〔xant(P我镨2)2 ]}(BR 2 = BCY 2(2),BBN(3); BBN = 9-硼杂双环[3.5.1]壬烷)和i PrNH 2是由于1的羟基配体的O–H键加到氨基硼烷的B–N键上的结果。在室温和CO气氛下,配合物2和3消除H 2,得到bor(II)-硼氧化物OsH(OBR 2)(CO)2 {κ2 - P,P - 〔xant(P我镨2)2 ]}(BR 2 = BCY 2(4),BBN(5))轴承κ 2 - P,P配位的醚-二膦。的二取代硼酸随后的还原消除- [R 2只BOH需要加热和长持续时间,并导致三羰基锇(0)衍生物OS(CO)3 {κ 2 - P,P - 〔xant(P我镨2)2 ]}(6),其中二膦的磷原子位于金属中心周围的供体原子的五角形金字塔的赤道平面内。在对比2和3,CO气氛下,前体1个消除水最初得到反式-dihydride OSH 2(CO){κ 3 - P,ö,P - 〔xant(P我镨2)2 ]}(7),其随后演变到顺-dihydride-顺二羰基衍生物OSH 2(CO)2 {κ 2- P,P - 〔xant(P我镨2)2 ]}(8),最后进入三羰基6。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号