当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oncogenic KRAS supports pancreatic cancer through regulation of nucleotide synthesis.
Nature Communications ( IF 14.7 ) Pub Date : 2018-11-23 , DOI: 10.1038/s41467-018-07472-8 Naiara Santana-Codina 1 , Anjali A Roeth 1, 2 , Yi Zhang 1 , Annan Yang 1 , Oksana Mashadova 3 , John M Asara 4 , Xiaoxu Wang 1 , Roderick T Bronson 5 , Costas A Lyssiotis 6, 7 , Haoqiang Ying 8 , Alec C Kimmelman 1, 9
Nature Communications ( IF 14.7 ) Pub Date : 2018-11-23 , DOI: 10.1038/s41467-018-07472-8 Naiara Santana-Codina 1 , Anjali A Roeth 1, 2 , Yi Zhang 1 , Annan Yang 1 , Oksana Mashadova 3 , John M Asara 4 , Xiaoxu Wang 1 , Roderick T Bronson 5 , Costas A Lyssiotis 6, 7 , Haoqiang Ying 8 , Alec C Kimmelman 1, 9
Affiliation
|
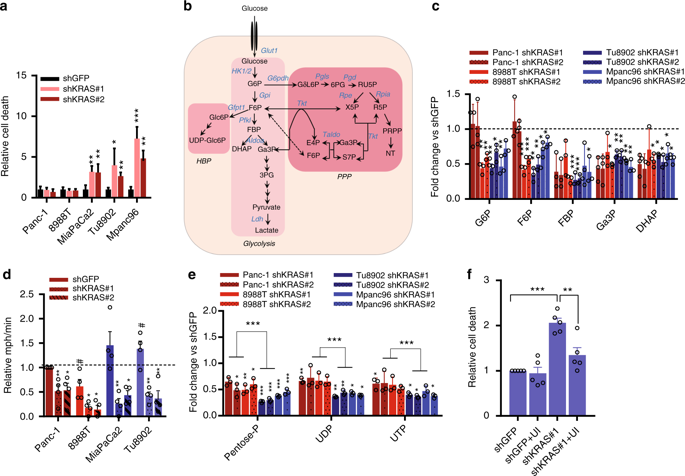
Oncogenic KRAS is the key driver of pancreatic ductal adenocarcinoma (PDAC). We previously described a role for KRAS in PDAC tumor maintenance through rewiring of cellular metabolism to support proliferation. Understanding the details of this metabolic reprogramming in human PDAC may provide novel therapeutic opportunities. Here we show that the dependence on oncogenic KRAS correlates with specific metabolic profiles that involve maintenance of nucleotide pools as key mediators of KRAS-dependence. KRAS promotes these effects by activating a MAPK-dependent signaling pathway leading to MYC upregulation and transcription of the non-oxidative pentose phosphate pathway (PPP) gene RPIA, which results in nucleotide biosynthesis. The use of MEK inhibitors recapitulates the KRAS-dependence pattern and the expected metabolic changes. Antagonizing the PPP or pyrimidine biosynthesis inhibits the growth of KRAS-resistant cells. Together, these data reveal differential metabolic rewiring between KRAS-resistant and sensitive cells, and demonstrate that targeting nucleotide metabolism can overcome resistance to KRAS/MEK inhibition.
中文翻译:

致癌性KRAS通过调节核苷酸合成来支持胰腺癌。
致癌性KRAS是胰腺导管腺癌(PDAC)的主要驱动力。我们先前描述了KRAS在PDAC肿瘤维持中的作用,方法是重新排列细胞代谢以支持增殖。了解人类PDAC中这种代谢重编程的细节可能会提供新的治疗机会。在这里,我们显示对致癌性KRAS的依赖性与特定的代谢谱相关,后者涉及维持核苷酸池作为KRAS依赖性的关键介体。KRAS通过激活MAPK依赖性信号传导途径来促进这些作用,从而导致MYC上调和非氧化性戊糖磷酸途径(PPP)基因RPIA的转录,从而导致核苷酸的生物合成。MEK抑制剂的使用概括了KRAS依赖性模式和预期的代谢变化。拮抗PPP或嘧啶的生物合成会抑制KRAS耐药细胞的生长。总之,这些数据揭示了耐KRAS的细胞与敏感细胞之间的代谢差异,并且证明了靶向核苷酸代谢可以克服对KRAS / MEK抑制的抵抗力。
更新日期:2018-11-24
中文翻译:

致癌性KRAS通过调节核苷酸合成来支持胰腺癌。
致癌性KRAS是胰腺导管腺癌(PDAC)的主要驱动力。我们先前描述了KRAS在PDAC肿瘤维持中的作用,方法是重新排列细胞代谢以支持增殖。了解人类PDAC中这种代谢重编程的细节可能会提供新的治疗机会。在这里,我们显示对致癌性KRAS的依赖性与特定的代谢谱相关,后者涉及维持核苷酸池作为KRAS依赖性的关键介体。KRAS通过激活MAPK依赖性信号传导途径来促进这些作用,从而导致MYC上调和非氧化性戊糖磷酸途径(PPP)基因RPIA的转录,从而导致核苷酸的生物合成。MEK抑制剂的使用概括了KRAS依赖性模式和预期的代谢变化。拮抗PPP或嘧啶的生物合成会抑制KRAS耐药细胞的生长。总之,这些数据揭示了耐KRAS的细胞与敏感细胞之间的代谢差异,并且证明了靶向核苷酸代谢可以克服对KRAS / MEK抑制的抵抗力。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号