Oncogenesis ( IF 5.9 ) Pub Date : 2018-11-23 , DOI: 10.1038/s41389-018-0101-3 Gi-Cheon Kim , Ho-Keun Kwon , Choong-Gu Lee , Ravi Verma , Dipayan Rudra , Taemook Kim , Keunsoo Kang , Jong Hee Nam , Young Kim , Sin-Hyeog Im
|
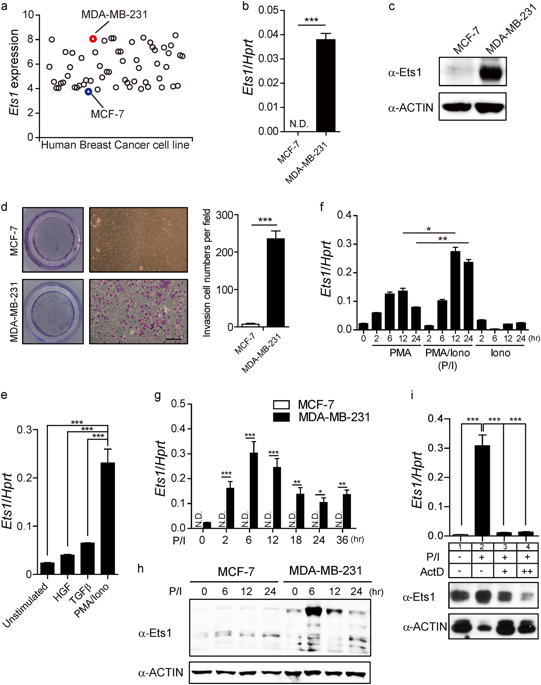
Breast cancer is highly aggressive and is the leading cause of cancer-related mortality in women in developed countries. The ETS proto-oncogene 1 (Ets1) has versatile roles during the cellular processes of cancer development. It is often highly expressed in breast cancers and mediates migration and invasion of human breast cancer cells. However, underlying mechanisms of Ets1 gene expression is still ambiguous. Here, we identified a core-regulatory element (CRE) located in the Ets1 promoter region (−540/−80 bp from TSS) that contains elements responsible for associating with NFATs and NF-κBs. Compared with the less metastatic breast cancer cells, metastatic breast cancer cells (MDA-MB-231) show open chromatin configurations in the CRE, which facilitates direct binding of NFATc2 and/or NFKB1/RELA complex to trans-activate Ets1 transcription. Moreover, enhanced level of Nfatc2 and Nfkb1 positively correlated with Ets1 expression in the human breast cancer specimens. Deletion of the CRE region by CRISPR/Cas9 system resulted in significant reduction in Ets1 expression, which led to alterations of Ets1-mediated transcription programs including tumor invasiveness-related genes. Proper regulation of Ets1 gene expression by targeting the NFATc2 and NFKB1/RELA interaction could be a potential therapeutic target for Ets1-mediated metastatic breast cancer.
中文翻译:

NFATc2和NFKB1 / RELA上调Ets1表达促进乳腺癌细胞侵袭性
乳腺癌具有很高的侵袭性,并且是发达国家女性与癌症相关的死亡率的主要原因。ETS原癌基因1(Ets1)在癌症发展的细胞过程中具有多种作用。它通常在乳腺癌中高表达,并介导人类乳腺癌细胞的迁移和侵袭。但是,Ets1的潜在机制基因表达仍然是模棱两可的。在这里,我们确定了位于Ets1启动子区域(距TSS为-540 / -80 bp)的核心调控元件(CRE),其中包含负责与NFAT和NF-κB关联的元件。与转移性较低的乳腺癌细胞相比,转移性乳腺癌细胞(MDA-MB-231)在CRE中显示出开放的染色质构型,这有助于NFATc2和/或NFKB1 / RELA复合物直接结合以反式激活Ets1转录。此外,增强的水平NFATC2和NFKB1与人类乳腺癌标本中的Ets1表达呈正相关。CRISPR / Cas9系统删除CRE区导致Ets1表达显着减少,从而导致Ets1介导的转录程序(包括肿瘤侵袭相关基因)发生变化。通过靶向NFATc2和NFKB1 / RELA相互作用正确调节Ets1基因表达可能是Ets1介导的转移性乳腺癌的潜在治疗靶点。












































 京公网安备 11010802027423号
京公网安备 11010802027423号