当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient Large Scale Syntheses of 3-Deoxy-d-manno-2-octulosonic acid (Kdo) and Its Derivatives
Organic Letters ( IF 4.9 ) Pub Date : 2015-04-30 00:00:00 , DOI: 10.1021/acs.orglett.5b00901 Yingle Feng 1 , Jie Dong 1 , Fangyuan Xu 1 , Aiyun Liu 1 , Li Wang 1 , Qi Zhang 1 , Yonghai Chai 1
Organic Letters ( IF 4.9 ) Pub Date : 2015-04-30 00:00:00 , DOI: 10.1021/acs.orglett.5b00901 Yingle Feng 1 , Jie Dong 1 , Fangyuan Xu 1 , Aiyun Liu 1 , Li Wang 1 , Qi Zhang 1 , Yonghai Chai 1
Affiliation
|
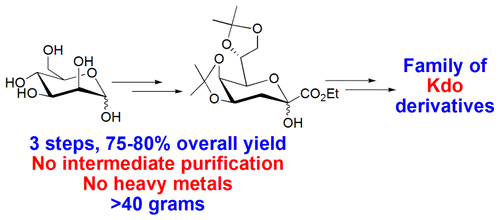
An efficient method to rapidly synthesize 3-deoxy-d-manno-2-octulosonic acid (Kdo) and its derivatives in large scale has been developed. Starting from d-mannose, the di-O-isopropylidene derivative of Kdo ethyl ester was prepared in three steps on a scale of more than 40 g in one batch in an overall yield of 75–80% without any intermediate purification. Kdo, Kdo glycal, and 2-acetylated Kdo ester were synthesized quickly in high yield from a di-O-isopropylidene derivative of Kdo ethyl ester. 2-Deoxy-β-Kdo ester was obtained with high stereoselectivity via the epimerization of the α-isomer using t-BuOH as a proton source.
中文翻译:

有效的大规模合成3-Deoxy-d-manno-2-octulosonic acid(Kdo)及其衍生物
快速高效的方法合成3-脱氧d -manno -2-辛酮糖酸(KDO)及其在大规模衍生物已被开发。从d-甘露糖开始,分三批以超过40 g的规模分三步制备Kdo乙酯的二-O-异亚丙基衍生物,总产率为75-80%,无需任何中间纯化。由Kdo乙酯的二-O-异亚丙基衍生物快速高产率地合成了Kdo,Kdo糖基和2-乙酰化的Kdo酯。通过使用t - BuOH作为质子源,通过α-异构体的差向异构化,以高立体选择性获得2-脱氧-β-Kdo酯。
更新日期:2015-04-30
中文翻译:

有效的大规模合成3-Deoxy-d-manno-2-octulosonic acid(Kdo)及其衍生物
快速高效的方法合成3-脱氧d -manno -2-辛酮糖酸(KDO)及其在大规模衍生物已被开发。从d-甘露糖开始,分三批以超过40 g的规模分三步制备Kdo乙酯的二-O-异亚丙基衍生物,总产率为75-80%,无需任何中间纯化。由Kdo乙酯的二-O-异亚丙基衍生物快速高产率地合成了Kdo,Kdo糖基和2-乙酰化的Kdo酯。通过使用t - BuOH作为质子源,通过α-异构体的差向异构化,以高立体选择性获得2-脱氧-β-Kdo酯。































 京公网安备 11010802027423号
京公网安备 11010802027423号