当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding LiOH Formation in a Li-O2 Battery with LiI and H2O Additives
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-11-20 00:00:00 , DOI: 10.1021/acscatal.8b02783 Tao Liu 1 , Gunwoo Kim 1, 2 , Erlendur Jónsson 1, 3 , Elizabeth Castillo-Martinez 1 , Israel Temprano 1 , Yuanlong Shao 1, 2 , Javier Carretero-González 1, 4 , Rachel N. Kerber 1 , Clare P. Grey 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-11-20 00:00:00 , DOI: 10.1021/acscatal.8b02783 Tao Liu 1 , Gunwoo Kim 1, 2 , Erlendur Jónsson 1, 3 , Elizabeth Castillo-Martinez 1 , Israel Temprano 1 , Yuanlong Shao 1, 2 , Javier Carretero-González 1, 4 , Rachel N. Kerber 1 , Clare P. Grey 1
Affiliation
|
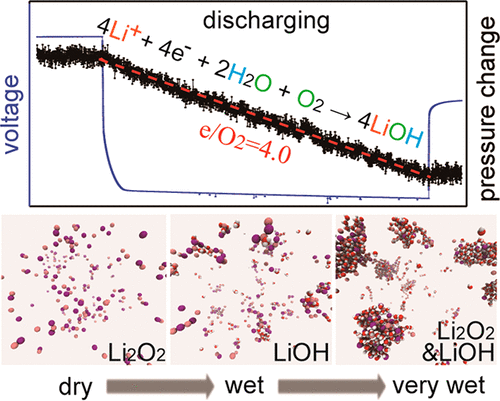
LiI-promoted LiOH formation in Li-O2 batteries with wet ether electrolytes has been investigated by Raman, nuclear magnetic resonance spectroscopy, operando pressure tests, and molecular dynamics simulations. We find that LiOH formation is a synergistic effect involving both H2O and LiI additives, whereas with either alone Li2O2 forms. LiOH is generated via a nominal four-electron oxygen reduction reaction, the hydrogen coming from H2O and the oxygen from both O2 and H2O, and with fewer side reactions than typically associated with Li2O2 formation; the presence of fewer parasitic reactions is attributed to the proton donor role of water, which can coordinate to O2– and the higher chemical stability of LiOH. Iodide plays a catalytic role in decomposing H2O2/HO2– and thereby promoting LiOH formation, its efficacy being highly dependent on the water concentration. This iodide catalysis becomes retarded at high water contents due to the formation of large water-solvated clusters, and Li2O2 forms again.
中文翻译:

了解具有LiI和H 2 O添加剂的Li-O 2电池中LiOH的形成
已通过拉曼光谱,核磁共振波谱,操作压力测试和分子动力学模拟研究了用湿醚电解质在Li-O 2电池中LiI促进的LiOH形成。我们发现,LiOH的形成是一种协同效应,涉及H 2 O和LiI添加剂,而单独使用Li 2 O 2形式。LiOH是通过名义上的四电子氧还原反应生成的,氢来自H 2 O,氧来自O 2和H 2 O,且副反应少于通常与Li 2 O 2相关的副反应。编队 更少的寄生反应的存在归因于水的质子供体的作用,其可以协调与O 2 -和LiOH的较高的化学稳定性。在碘化物分解ħ起着促进作用2 ö 2 / HO 2 -和由此促进形成的LiOH,其效能是高度依赖于水浓度。由于形成较大的水溶簇,该碘化物催化在高水含量下变得迟滞,并且再次形成Li 2 O 2。
更新日期:2018-11-20
中文翻译:

了解具有LiI和H 2 O添加剂的Li-O 2电池中LiOH的形成
已通过拉曼光谱,核磁共振波谱,操作压力测试和分子动力学模拟研究了用湿醚电解质在Li-O 2电池中LiI促进的LiOH形成。我们发现,LiOH的形成是一种协同效应,涉及H 2 O和LiI添加剂,而单独使用Li 2 O 2形式。LiOH是通过名义上的四电子氧还原反应生成的,氢来自H 2 O,氧来自O 2和H 2 O,且副反应少于通常与Li 2 O 2相关的副反应。编队 更少的寄生反应的存在归因于水的质子供体的作用,其可以协调与O 2 -和LiOH的较高的化学稳定性。在碘化物分解ħ起着促进作用2 ö 2 / HO 2 -和由此促进形成的LiOH,其效能是高度依赖于水浓度。由于形成较大的水溶簇,该碘化物催化在高水含量下变得迟滞,并且再次形成Li 2 O 2。















































 京公网安备 11010802027423号
京公网安备 11010802027423号