当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Engineering of Bacillus paralicheniformis Acid Urease To Degrade Urea and Ethyl Carbamate in Model Chinese Rice Wine
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2018-11-19 00:00:00 , DOI: 10.1021/acs.jafc.8b04566 Qingtao Liu , Xinhui Yao , Qixing Liang , Jianghua Li , Fang Fang , Guocheng Du , Zhen Kang
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2018-11-19 00:00:00 , DOI: 10.1021/acs.jafc.8b04566 Qingtao Liu , Xinhui Yao , Qixing Liang , Jianghua Li , Fang Fang , Guocheng Du , Zhen Kang
|
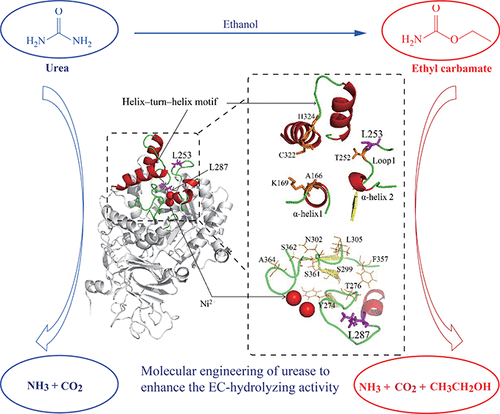
Bacillus paralicheniformis urease (BpUrease) has been shown to be a promising biocatalyst for degrading the carcinogenic chemical ethyl carbamate (EC or urethane) in rice wine. However, low EC affinity and catalytic efficiency limit the practical application of BpUrease. In this study, we improved the EC degradation capability of BpUrease by site-saturation mutagenesis (SSM). The best variant L253P/L287N showed a 49% increase in EC affinity, 1027% increase in catalytic efficiency (kcat/Km), and 583% increase in half-life (t1/2) at 70 °C. Homology modeling analysis suggest that mutation of Leu253 to Pro increased the BpUrease EC specificity by affecting the interaction between Arg339 with the catalytic residue His323, while Leu287Asn mutation benefits EC specificity and affinity by changing the interaction networks among the residues in the catalytic pocket. Our results show that the L253P/L287N variant efficiently degraded urea and EC in a model rice wine, making it a good candidate for practical application in the food industry.
中文翻译:

副粘芽孢杆菌酸尿素酶降解典型黄酒中尿素和氨基甲酸乙酯的分子工程
副芽孢杆菌脲酶(BpUrease)已被证明是降解黄酒中致癌化学氨基甲酸乙酯(EC或尿烷)的有前途的生物催化剂。但是,低EC亲和力和催化效率限制了BpUrease的实际应用。在这项研究中,我们通过位点饱和诱变(SSM)提高了BpUrease的EC降解能力。最佳变体L253P / L287N的EC亲和力增加49%,催化效率(k cat / K m)增加1027%,半衰期增加583%(t 1/2))在70°C下。同源性模型分析表明,Leu253突变为Pro可通过影响Arg339与催化残基His323之间的相互作用来提高BpUrease EC特异性,而Leu287Asn突变可通过改变催化口袋中残基之间的相互作用网络来提高EC特异性和亲和力。我们的结果表明,L253P / L287N变体可有效降解模型米酒中的尿素和EC,使其成为食品工业中实际应用的良好候选者。
更新日期:2018-11-19
中文翻译:

副粘芽孢杆菌酸尿素酶降解典型黄酒中尿素和氨基甲酸乙酯的分子工程
副芽孢杆菌脲酶(BpUrease)已被证明是降解黄酒中致癌化学氨基甲酸乙酯(EC或尿烷)的有前途的生物催化剂。但是,低EC亲和力和催化效率限制了BpUrease的实际应用。在这项研究中,我们通过位点饱和诱变(SSM)提高了BpUrease的EC降解能力。最佳变体L253P / L287N的EC亲和力增加49%,催化效率(k cat / K m)增加1027%,半衰期增加583%(t 1/2))在70°C下。同源性模型分析表明,Leu253突变为Pro可通过影响Arg339与催化残基His323之间的相互作用来提高BpUrease EC特异性,而Leu287Asn突变可通过改变催化口袋中残基之间的相互作用网络来提高EC特异性和亲和力。我们的结果表明,L253P / L287N变体可有效降解模型米酒中的尿素和EC,使其成为食品工业中实际应用的良好候选者。













































 京公网安备 11010802027423号
京公网安备 11010802027423号