当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Redox Properties of Cu2O(100) and (111) Surfaces
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-12-05 , DOI: 10.1021/acs.jpcc.8b08494 Chunlei Wang 1 , Heloise Tissot 1 , Carlos Escudero 2 , Virginia Pérez-Dieste 2 , Dario Stacchiola 3 , Jonas Weissenrieder 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-12-05 , DOI: 10.1021/acs.jpcc.8b08494 Chunlei Wang 1 , Heloise Tissot 1 , Carlos Escudero 2 , Virginia Pérez-Dieste 2 , Dario Stacchiola 3 , Jonas Weissenrieder 1
Affiliation
|
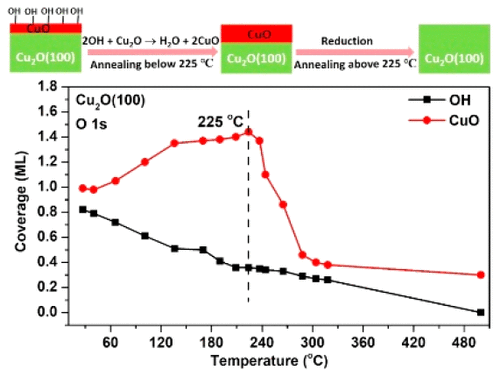
Intense research efforts are directed toward Cu and Cu2O based catalysts as they are viewed as potential replacements for noble metal catalysts. However, applications are hampered by deactivation, e.g., through facile complete oxidation to CuO. Despite the importance of the redox processes for Cu2O catalysts, a molecular level understanding of the deactivation process is still lacking. Here we study the initial stages of oxidization of well-defined Cu2O bulk single crystals of (100) and (111) termination by means of synchrotron radiation X-ray photoemission spectroscopy (XPS) and scanning tunneling microscopy (STM). Exposure of the (100) surface to 1 mbar O2 at 25 °C results in the formation of a 1.0 monolayer (ML) CuO surface oxide. The surface is covered by 0.7 ML OH groups from trace moisture in the reaction gas. In contrast, neither hydroxylation nor oxidation was observed on the (111) surface under similar mild exposure conditions. On Cu2O(111) the initial formation of CuO requires annealing to ∼400 °C in 1 mbar O2, highlighting the markedly different reactivity of the two Cu2O surfaces. Annealing of the (100) surface, under ultrahigh vacuum conditions, to temperatures up to ∼225 °C resulted in removal of the OH groups (0.46 ML decrease) at a rate similar to a detected increase in CuO coverage (0.45 ML increase), suggesting the reaction path 2OHadsorbed + Cu2Osolid → H2Ogas + 2CuOsolid. STM was used to correlate the observed changes in surface chemistry with surface morphology, confirming the surface hydroxylation and CuO formation. The STM analysis showed dramatic changes in surface morphology demonstrating a high mobility of the active species under reaction conditions.
中文翻译:

Cu 2 O(100)和(111)表面的氧化还原特性
大量的研究工作针对基于Cu和Cu 2 O的催化剂,因为它们被视为贵金属催化剂的潜在替代品。然而,应用由于失活而受到阻碍,例如通过容易地完全氧化成CuO而受到阻碍。尽管氧化还原方法对Cu 2 O催化剂很重要,但仍缺乏对钝化过程的分子水平的了解。在这里,我们通过同步辐射X射线光电子能谱(XPS)和扫描隧道显微镜(STM)研究了(100)和(111)终止的定义明确的Cu 2 O块状单晶的氧化初期。(100)表面暴露于1 mbar O 2在25°C下,会形成1.0单层(ML)CuO表面氧化物。反应气体中的微量水分覆盖了0.7 ML OH基团的表面。相反,在相似的温和曝光条件下,在(111)表面上未观察到羟基化或氧化。在Cu 2 O(111)上,CuO的初始形成需要在1 mbar O 2中退火至约400°C ,这突出了两个Cu 2 O表面的显着不同的反应性。在超高真空条件下将(100)表面退火到最高约225°C的温度导致以检测到的CuO覆盖率增加(0.45 ML增加)的速率除去OH基团(减少0.46 ML),提示反应路径2OH吸附+ Cu 2O固体→H 2 O气+ 2CuO固体。使用STM将观察到的表面化学变化与表面形态相关联,确认了表面羟基化和CuO的形成。STM分析表明,表面形态发生了巨大变化,表明活性物质在反应条件下具有很高的迁移率。
更新日期:2018-12-06
中文翻译:

Cu 2 O(100)和(111)表面的氧化还原特性
大量的研究工作针对基于Cu和Cu 2 O的催化剂,因为它们被视为贵金属催化剂的潜在替代品。然而,应用由于失活而受到阻碍,例如通过容易地完全氧化成CuO而受到阻碍。尽管氧化还原方法对Cu 2 O催化剂很重要,但仍缺乏对钝化过程的分子水平的了解。在这里,我们通过同步辐射X射线光电子能谱(XPS)和扫描隧道显微镜(STM)研究了(100)和(111)终止的定义明确的Cu 2 O块状单晶的氧化初期。(100)表面暴露于1 mbar O 2在25°C下,会形成1.0单层(ML)CuO表面氧化物。反应气体中的微量水分覆盖了0.7 ML OH基团的表面。相反,在相似的温和曝光条件下,在(111)表面上未观察到羟基化或氧化。在Cu 2 O(111)上,CuO的初始形成需要在1 mbar O 2中退火至约400°C ,这突出了两个Cu 2 O表面的显着不同的反应性。在超高真空条件下将(100)表面退火到最高约225°C的温度导致以检测到的CuO覆盖率增加(0.45 ML增加)的速率除去OH基团(减少0.46 ML),提示反应路径2OH吸附+ Cu 2O固体→H 2 O气+ 2CuO固体。使用STM将观察到的表面化学变化与表面形态相关联,确认了表面羟基化和CuO的形成。STM分析表明,表面形态发生了巨大变化,表明活性物质在反应条件下具有很高的迁移率。































 京公网安备 11010802027423号
京公网安备 11010802027423号