当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
2-(Naphthalen-1-yl)thiophene as a New Motif for Porphyrinoids: Meso-Fused Carbaporphyrin
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-03-02 , DOI: 10.1021/jacs.6b01063 Jung-Ho Hong 1 , Adil S. Aslam 1 , Masatoshi Ishida 2 , Shigeki Mori 3 , Hiroyuki Furuta 2 , Dong-Gyu Cho 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-03-02 , DOI: 10.1021/jacs.6b01063 Jung-Ho Hong 1 , Adil S. Aslam 1 , Masatoshi Ishida 2 , Shigeki Mori 3 , Hiroyuki Furuta 2 , Dong-Gyu Cho 1
Affiliation
|
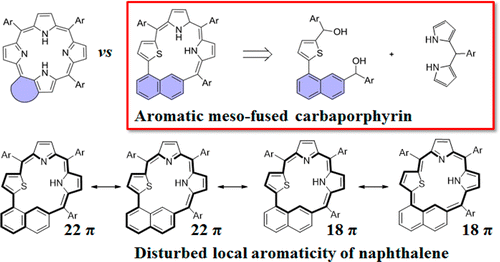
The first synthesis of meso-fused carbaporphyrin via a premodification method was accomplished by substituting two pyrrole moieties and one meso-carbon with 2-(naphthalen-1-yl)thiophene. The obtained global π-conjugation pathway of the macrocycle noticeably disturbs the 10π local aromaticity of naphthalene, and its aromatic nature was supported by NMR spectroscopy together with nucleus-independent chemical shift, anisotropy of the induced current density, and harmonic oscillator stabilization energy calculations. In addition, the meso-fused carbaporphyrin also allowed the formation of a square planar Pd(II) complex.
中文翻译:

2-(Naphthalen-1-yl) 噻吩作为类卟啉的新基序:介晶稠合碳卟啉
通过用 2-(naphthalen-1-yl) 噻吩取代两个吡咯部分和一个中间碳,通过预修饰方法首次合成内消旋稠合碳卟啉。获得的大环全局 π 共轭通路显着扰乱了萘的 10π 局部芳香性,其芳香性得到了核磁共振光谱以及与核无关的化学位移、感应电流密度的各向异性和谐振子稳定能量计算的支持。此外,介孔融合的碳卟啉还允许形成方形平面 Pd(II) 复合物。
更新日期:2016-03-02
中文翻译:

2-(Naphthalen-1-yl) 噻吩作为类卟啉的新基序:介晶稠合碳卟啉
通过用 2-(naphthalen-1-yl) 噻吩取代两个吡咯部分和一个中间碳,通过预修饰方法首次合成内消旋稠合碳卟啉。获得的大环全局 π 共轭通路显着扰乱了萘的 10π 局部芳香性,其芳香性得到了核磁共振光谱以及与核无关的化学位移、感应电流密度的各向异性和谐振子稳定能量计算的支持。此外,介孔融合的碳卟啉还允许形成方形平面 Pd(II) 复合物。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号