Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2018-11-19 , DOI: 10.1038/s41594-018-0152-3 Meghna Kataria 1, 2 , Stephane Mouilleron 3 , Moon-Hyeong Seo 4, 5 , Carles Corbi-Verge 4 , Philip M Kim 4, 6 , Frank Uhlmann 1
|
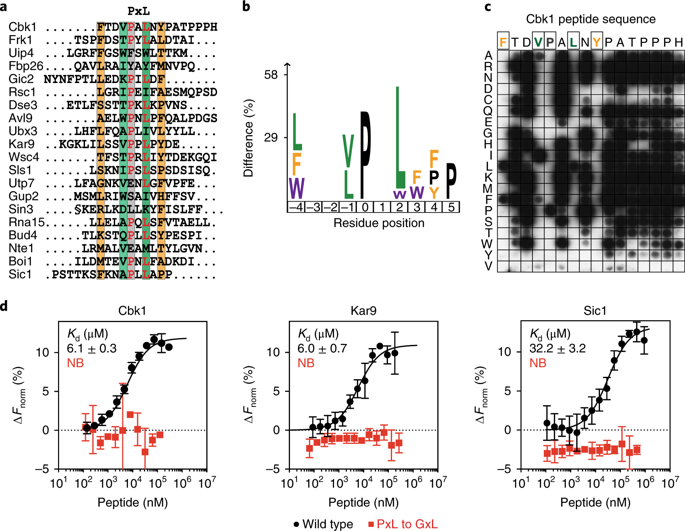
The cell division cycle consists of a series of temporally ordered events. Cell cycle kinases and phosphatases provide key regulatory input, but how the correct substrate phosphorylation and dephosphorylation timing is achieved is incompletely understood. Here we identify a PxL substrate recognition motif that instructs dephosphorylation by the budding yeast Cdc14 phosphatase during mitotic exit. The PxL motif was prevalent in Cdc14-binding peptides enriched in a phage display screen of native disordered protein regions. PxL motif removal from the Cdc14 substrate Cbk1 delays its dephosphorylation, whereas addition of the motif advances dephosphorylation of otherwise late Cdc14 substrates. Crystal structures of Cdc14 bound to three PxL motif substrate peptides provide a molecular explanation for PxL motif recognition on the phosphatase surface. Our results illustrate the sophistication of phosphatase–substrate interactions and identify them as an important determinant of ordered cell cycle progression.
中文翻译:

PxL 基序通过 Cdc14 磷酸酶促进及时的细胞周期底物去磷酸化
细胞分裂周期由一系列按时间顺序排列的事件组成。细胞周期激酶和磷酸酶提供关键的调节输入,但如何实现正确的底物磷酸化和去磷酸化时间尚不完全清楚。在这里,我们确定了一个 PxL 底物识别基序,该基序指示出芽酵母 Cdc14 磷酸酶在有丝分裂退出期间进行去磷酸化。PxL 基序在富含天然无序蛋白质区域的噬菌体展示屏幕中的 Cdc14 结合肽中普遍存在。从 Cdc14 底物 Cbk1 中去除 PxL 基序会延迟其去磷酸化,而添加基序会促进其他晚期 Cdc14 底物的去磷酸化。与三个 PxL 基序底物肽结合的 Cdc14 的晶体结构为磷酸酶表面上的 PxL 基序识别提供了分子解释。































 京公网安备 11010802027423号
京公网安备 11010802027423号