当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Clinical Candidate BMS-823778 as an Inhibitor of Human 11β-Hydroxysteroid Dehydrogenase Type 1 (11β-HSD-1).
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2018-11-13 , DOI: 10.1021/acsmedchemlett.8b00307 Jun Li 1 , Lawrence J Kennedy 1 , Steven J Walker 1 , Haixia Wang 1 , James J Li 1 , Zhenqiu Hong 1 , Stephen P O'Connor 1 , Xiang-Yang Ye 1 , Stephanie Chen 1 , Shung Wu 1 , David S Yoon 1 , Akbar Nayeem 2 , Daniel M Camac 2 , Vidhyashankar Ramamurthy 2 , Paul E Morin 2 , Steven Sheriff 2 , Mengmeng Wang 1 , Timothy W Harper 1 , Rajasree Golla 1 , Ramakrishna Seethala 1 , Thomas Harrity 1 , Randolph P Ponticiello 1 , Nathan N Morgan 1 , Joseph R Taylor 1 , Rachel Zebo 1 , Brad Maxwell 2 , Frederick Moulin 1 , David A Gordon 1 , Jeffrey A Robl 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2018-11-13 , DOI: 10.1021/acsmedchemlett.8b00307 Jun Li 1 , Lawrence J Kennedy 1 , Steven J Walker 1 , Haixia Wang 1 , James J Li 1 , Zhenqiu Hong 1 , Stephen P O'Connor 1 , Xiang-Yang Ye 1 , Stephanie Chen 1 , Shung Wu 1 , David S Yoon 1 , Akbar Nayeem 2 , Daniel M Camac 2 , Vidhyashankar Ramamurthy 2 , Paul E Morin 2 , Steven Sheriff 2 , Mengmeng Wang 1 , Timothy W Harper 1 , Rajasree Golla 1 , Ramakrishna Seethala 1 , Thomas Harrity 1 , Randolph P Ponticiello 1 , Nathan N Morgan 1 , Joseph R Taylor 1 , Rachel Zebo 1 , Brad Maxwell 2 , Frederick Moulin 1 , David A Gordon 1 , Jeffrey A Robl 1
Affiliation
|
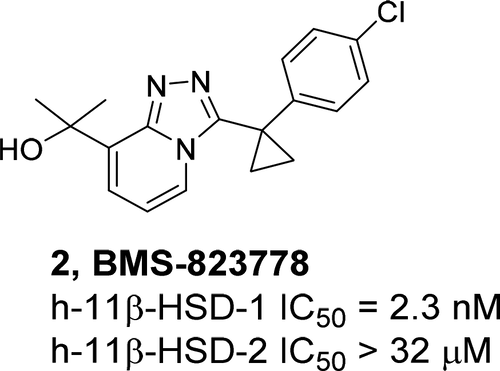
BMS-823778 (2), a 1,2,4-triazolopyridinyl-methanol derived analog, was identified as a potent and selective inhibitor of human 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD-1) enzyme (IC50 = 2.3 nM) with >10,000-fold selectivity over 11β-HSD-2. Compound 2 exhibits robust acute pharmacodynamic effects in cynomolgus monkeys (ED50 = 0.6 mg/kg) and in diet-induced obese (DIO) mice (ED50 = 34 mg/kg). Compound 2 also showed excellent inhibition in an ex vivo adipose DIO mouse model (ED50 = 5.2 mg/kg). Oral bioavailability ranges from 44% to 100% in preclinical species. Its favorable development properties, pharmacokinetics, high adipose-to-plasma concentration ratio, and preclinical pharmacology profile have prompted the evaluation of 2 for the treatment of type 2 diabetes and metabolic syndrome in phase 2 clinical trials.
中文翻译:

发现作为人11β-羟基类固醇脱氢酶1(11β-HSD-1)抑制剂的临床候选药物BMS-823778。
BMS-823778(2)是一种1,2,4-三唑并吡啶基-甲醇衍生的类似物,被确定为1β型11β-羟类固醇脱氢酶(11β-HSD-1)酶的强效和选择性抑制剂(IC50 = 2.3 nM)相对于11β-HSD-2具有> 10,000倍的选择性。化合物2在食蟹猴(ED50 = 0.6 mg / kg)和饮食诱发的肥胖(DIO)小鼠(ED50 = 34 mg / kg)中表现出强大的急性药效作用。化合物2在离体脂肪DIO小鼠模型中也显示出优异的抑制作用(ED50 = 5.2 mg / kg)。临床前物种的口服生物利用度范围为44%至100%。它具有良好的开发性能,药代动力学,高的脂肪对血浆浓度比以及临床前药理学特性,促使2期临床试验对2型糖尿病和代谢综合征的治疗进行了评估。
更新日期:2018-11-13
中文翻译:

发现作为人11β-羟基类固醇脱氢酶1(11β-HSD-1)抑制剂的临床候选药物BMS-823778。
BMS-823778(2)是一种1,2,4-三唑并吡啶基-甲醇衍生的类似物,被确定为1β型11β-羟类固醇脱氢酶(11β-HSD-1)酶的强效和选择性抑制剂(IC50 = 2.3 nM)相对于11β-HSD-2具有> 10,000倍的选择性。化合物2在食蟹猴(ED50 = 0.6 mg / kg)和饮食诱发的肥胖(DIO)小鼠(ED50 = 34 mg / kg)中表现出强大的急性药效作用。化合物2在离体脂肪DIO小鼠模型中也显示出优异的抑制作用(ED50 = 5.2 mg / kg)。临床前物种的口服生物利用度范围为44%至100%。它具有良好的开发性能,药代动力学,高的脂肪对血浆浓度比以及临床前药理学特性,促使2期临床试验对2型糖尿病和代谢综合征的治疗进行了评估。






























 京公网安备 11010802027423号
京公网安备 11010802027423号