当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reconciling Structural and Spectroscopic Fingerprints of the Oxygen-Evolving Complex of Photosystem II: A Computational Study of the S2 State
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-11-28 , DOI: 10.1021/acs.jpcb.8b08147 He Chen , David A. Case , G. Charles Dismukes
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-11-28 , DOI: 10.1021/acs.jpcb.8b08147 He Chen , David A. Case , G. Charles Dismukes
|
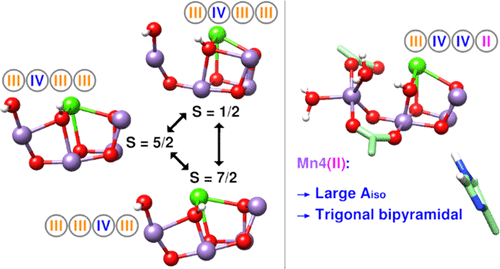
The catalytic cycle of photosynthetic water oxidation occurs at the Mn4CaO5 oxygen-evolving complex (OEC) of photosystem II. Extensive spectroscopic data have been collected on the intermediates, especially the S2 (Kok) state, although the proton and electron inventories (Mn oxidation states) are still uncertain. The “high oxidation” paradigm assigns S2 Mn oxidation level (III, IV, IV, IV) or (IV, IV, IV, III), whereas a “low oxidation” paradigm posits two additional electrons. Here, we investigate the geometric (X-ray diffraction, extended X-ray absorption fine structure) and spectroscopic (electron paramagnetic resonance (EPR), electron nuclear double resonance (ENDOR)) properties of the S2 state using quantum chemical density functional theory calculations, focusing on the neglected low paradigm. Two interconvertible electronic spin configurations are predicted as ground states, producing multiline (S = 1/2) and broad (S = 5/2) EPR signals in the low paradigm oxidation state (III, IV, III, III) and with W2 as OH– and O5 as OH–. They have “open” (S = 5/2) and “closed” (S = 1/2) Mn3CaO4-cubane geometries. Other energetically accessible isomers with ground spin states 1/2, 7/2, 9/2, or 11/2 can be obtained through perturbations of hydrogen-bonding networks (e.g., H+ from His337 to O3 or W2), consistent with experimental observations. Conformers with the low oxidation state configuration (III, IV, IV, II) also become energetically accessible when the protonation states are O5 (OH–), W2 (H2O), and neutral His337. The configuration with (III, IV, III, III) agrees well with earlier low-temperature EPR and ENDOR interpretations, whereas the MnII-containing configuration agrees partially with recent ENDOR data. However, the low oxidation paradigm does not yield isotropic ligand hyperfine interactions in good agreement with observed values. We conclude that the low Mn oxidation state proposal for the OEC can closely fit most of the available structural and electronic data for S2 at accessible energies.
中文翻译:

调和光系统II析氧复合物的结构和光谱指纹:S 2状态的计算研究
光合水氧化的催化循环发生在光系统II的Mn 4 CaO 5析氧复合物(OEC)处。尽管质子和电子存量(Mn氧化态)仍不确定,但已收集了有关中间体的大量光谱数据,尤其是S 2(Kok)状态。“高氧化”范式指定S 2 Mn的氧化水平为(III,IV,IV,IV)或(IV,IV,IV,III),而“低氧化”范式则附加了两个电子。在这里,我们研究了S 2的几何(X射线衍射,扩展的X射线吸收精细结构)和光谱(电子顺磁共振(EPR),电子核双共振(ENDOR))特性利用量子化学密度泛函理论计算态,重点放在被忽略的低范式上。两种可互转换的电子自旋结构被预测为基态,在低范式氧化态(III,IV,III,III)和W2分别产生多线(S = 1/2)和宽(S = 5/2)EPR信号OH –和O5为OH –。它们具有“开放”(S = 5/2)和“封闭”(S = 1/2)Mn 3 CaO 4-古巴几何形状。可以通过氢键网络的扰动(例如H +(从His337到O3或W2),与实验观察结果一致。当质子化态为O5(OH -),W2(H 2 O)和中性His337时,具有低氧化态构型(III,IV,IV,II)的异构体也可以通过能量途径获得。(III,IV,III,III)的构型与早期的低温EPR和ENDOR解释非常吻合,而含Mn II的构型与最近的ENDOR数据部分吻合。但是,低氧化范式不会产生与观察值良好吻合的各向同性配体超精细相互作用。我们得出结论,针对OEC的低Mn氧化态提议可以非常适合S 2的大多数可用结构和电子数据 以可及的能量。
更新日期:2018-11-29
中文翻译:

调和光系统II析氧复合物的结构和光谱指纹:S 2状态的计算研究
光合水氧化的催化循环发生在光系统II的Mn 4 CaO 5析氧复合物(OEC)处。尽管质子和电子存量(Mn氧化态)仍不确定,但已收集了有关中间体的大量光谱数据,尤其是S 2(Kok)状态。“高氧化”范式指定S 2 Mn的氧化水平为(III,IV,IV,IV)或(IV,IV,IV,III),而“低氧化”范式则附加了两个电子。在这里,我们研究了S 2的几何(X射线衍射,扩展的X射线吸收精细结构)和光谱(电子顺磁共振(EPR),电子核双共振(ENDOR))特性利用量子化学密度泛函理论计算态,重点放在被忽略的低范式上。两种可互转换的电子自旋结构被预测为基态,在低范式氧化态(III,IV,III,III)和W2分别产生多线(S = 1/2)和宽(S = 5/2)EPR信号OH –和O5为OH –。它们具有“开放”(S = 5/2)和“封闭”(S = 1/2)Mn 3 CaO 4-古巴几何形状。可以通过氢键网络的扰动(例如H +(从His337到O3或W2),与实验观察结果一致。当质子化态为O5(OH -),W2(H 2 O)和中性His337时,具有低氧化态构型(III,IV,IV,II)的异构体也可以通过能量途径获得。(III,IV,III,III)的构型与早期的低温EPR和ENDOR解释非常吻合,而含Mn II的构型与最近的ENDOR数据部分吻合。但是,低氧化范式不会产生与观察值良好吻合的各向同性配体超精细相互作用。我们得出结论,针对OEC的低Mn氧化态提议可以非常适合S 2的大多数可用结构和电子数据 以可及的能量。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号