当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cucurbituril-Mediated Catalytic Hydrolysis: A Kinetic and Computational Study with Neutral and Cationic Dioxolanes in CB7
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-11-15 00:00:00 , DOI: 10.1021/acscatal.8b03605 Leandro Scorsin 1 , Juliano A. Roehrs 2 , Roberta R. Campedelli 1 , Giovanni F. Caramori 1 , Alexandre O. Ortolan 1 , Renato L. T. Parreira 3 , Haidi D. Fiedler 1 , Angel Acuña 4 , Luis García-Río 4 , Faruk Nome 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-11-15 00:00:00 , DOI: 10.1021/acscatal.8b03605 Leandro Scorsin 1 , Juliano A. Roehrs 2 , Roberta R. Campedelli 1 , Giovanni F. Caramori 1 , Alexandre O. Ortolan 1 , Renato L. T. Parreira 3 , Haidi D. Fiedler 1 , Angel Acuña 4 , Luis García-Río 4 , Faruk Nome 1
Affiliation
|
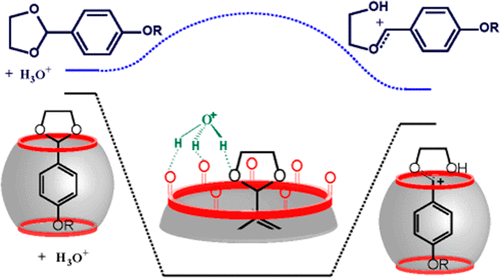
Cucurbit[7]uril (CB7) catalyzes the acid hydrolysis of alkoxyphenyldioxolanes bearing both neutral and cationic alkoxy groups. The magnitude of the catalytic effect depends on the dioxolane structure, as reflected by both the CB7 binding constants and the catalysis rate constants. However, there is no clear relationship in such a way that increasing the binding affinity (cationic dioxolanes or large alkoxy groups) does not enhance the catalytic effect. The A-1 mechanism for dioxolane hydrolysis involves the protonation and formation of a carbocation by protonated dioxolane ring opening. Supramolecular catalysis takes place through the formation of the ternary complex [email protected]CB7@H3O+, where the hydronium ion is stabilized by hydrogen bonding with the carbonyl groups of the CB7 portal. The ternary complex evolves to a binary complex by protonation of dioxolane and release of a water molecule. It is important to note that these structures are only stable in the presence of CB7 and not in bulk water. The carbocation is formed by opening the protonated dioxolane group in the rate-determining step. The distance between the carbonyl portal of CB7 and the dioxolane group in the ternary and binary complexes (protonated and carbocation) increases with the alkyl chain length, resulting in the loss of the CB7 stabilizing effect and decrease in catalytic efficiency. The existence of two recognition motifs with cationic dioxolanes results in the formation of both 1:1 and 2:1 complexes with different catalytic properties.
中文翻译:

葫芦素介导的催化水解:中性和阳离子二氧戊环在CB7中的动力学和计算研究。
葫芦[7] uril(CB7)催化带有中性和阳离子烷氧基的烷氧基苯基二氧戊环的酸水解。催化效果的大小取决于二氧戊环的结构,如CB7结合常数和催化速率常数所反映。但是,没有明显的关系,即增加结合亲和力(阳离子二氧戊环或较大的烷氧基)不会增强催化作用。二氧戊环水解的A-1机制涉及质子化和通过质子化的二氧戊环开环形成碳正离子。超分子催化通过三元络合物的形成而发生[受电子邮件保护] CB7 @H 3 O +,其中水合氢氧根离子通过与CB7门户的羰基进行氢键键合而稳定。通过二氧戊环的质子化和水分子的释放,三元络合物演变为二元络合物。重要的是要注意,这些结构仅在存在CB7的情况下才稳定,而在大量水中则不稳定。碳阳离子是通过在速率确定步骤中打开质子化的二氧戊环基团而形成的。CB7的羰基门户与三元和二元配合物(质子化和碳阳离子化)中的二氧戊环基团之间的距离随烷基链长的增加而增加,导致CB7的损失稳定作用并降低催化效率。带有阳离子二氧戊环的两个识别基元的存在导致形成具有不同催化特性的1:1和2:1配合物。
更新日期:2018-11-15
中文翻译:

葫芦素介导的催化水解:中性和阳离子二氧戊环在CB7中的动力学和计算研究。
葫芦[7] uril(CB7)催化带有中性和阳离子烷氧基的烷氧基苯基二氧戊环的酸水解。催化效果的大小取决于二氧戊环的结构,如CB7结合常数和催化速率常数所反映。但是,没有明显的关系,即增加结合亲和力(阳离子二氧戊环或较大的烷氧基)不会增强催化作用。二氧戊环水解的A-1机制涉及质子化和通过质子化的二氧戊环开环形成碳正离子。超分子催化通过三元络合物的形成而发生[受电子邮件保护] CB7 @H 3 O +,其中水合氢氧根离子通过与CB7门户的羰基进行氢键键合而稳定。通过二氧戊环的质子化和水分子的释放,三元络合物演变为二元络合物。重要的是要注意,这些结构仅在存在CB7的情况下才稳定,而在大量水中则不稳定。碳阳离子是通过在速率确定步骤中打开质子化的二氧戊环基团而形成的。CB7的羰基门户与三元和二元配合物(质子化和碳阳离子化)中的二氧戊环基团之间的距离随烷基链长的增加而增加,导致CB7的损失稳定作用并降低催化效率。带有阳离子二氧戊环的两个识别基元的存在导致形成具有不同催化特性的1:1和2:1配合物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号