当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Earth-Abundant Oxygen Electrocatalysts for Alkaline Anion-Exchange-Membrane Water Electrolysis: Effects of Catalyst Conductivity and Comparison with Performance in Three-Electrode Cells
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-11-15 00:00:00 , DOI: 10.1021/acscatal.8b04001 Dongyu Xu 1, 2 , Michaela Burke Stevens 1 , Monty R. Cosby 1 , Sebastian Z. Oener 1 , Adam M. Smith 1 , Lisa J. Enman 1 , Katherine E. Ayers 3 , Christopher B. Capuano 3 , Julie N. Renner 4 , Nemanja Danilovic 5 , Yaogang Li 2 , Hongzhi Wang 2 , Qinghong Zhang 2 , Shannon W. Boettcher 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-11-15 00:00:00 , DOI: 10.1021/acscatal.8b04001 Dongyu Xu 1, 2 , Michaela Burke Stevens 1 , Monty R. Cosby 1 , Sebastian Z. Oener 1 , Adam M. Smith 1 , Lisa J. Enman 1 , Katherine E. Ayers 3 , Christopher B. Capuano 3 , Julie N. Renner 4 , Nemanja Danilovic 5 , Yaogang Li 2 , Hongzhi Wang 2 , Qinghong Zhang 2 , Shannon W. Boettcher 1
Affiliation
|
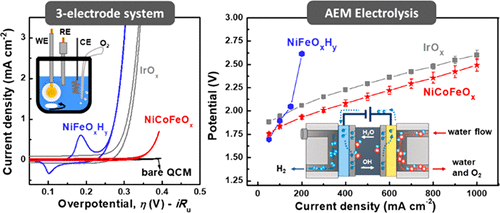
Anion exchange membrane (AEM) electrolysis is a promising technology to produce hydrogen through the splitting of pure water. In contrast to proton-exchange-membrane (PEM) technology, which requires precious-metal oxide anodes, AEM systems allow for the use of earth-abundant anode catalysts. Here we report a study of first-row transition-metal (oxy)hydroxide/oxide catalyst powders for application in AEM devices and compare physical properties and performance to benchmark IrOx catalysts as well as typical catalysts for alkaline electrolyzers. We show that the catalysts’ oxygen-evolution activity measured in alkaline electrolyte using a typical three-electrode cell is a poor indicator of performance in the AEM system. The best oxygen-evolution-reaction (OER) catalysts in alkaline electrolyte, NiFeOxHy oxyhydroxides, are the worst in AEM electrolysis devices where a solid alkaline electrolyte is used along with a pure water feed. NiCoOx-based catalysts show the best performance in AEM electrolysis. The performance can be further improved by adding Fe species to the particle surface. We attribute the differences in performance in part to differences in the electrical conductivity of the catalyst phases, which are also measured and reported.
中文翻译:

碱性阴离子交换膜水电解中的大地富氧电催化剂:催化剂电导率的影响以及三电极电池性能的比较
阴离子交换膜(AEM)电解是一种有希望的技术,它可以通过分解纯水来生产氢气。与质子交换膜(PEM)技术需要贵金属氧化物阳极相反,AEM系统允许使用富含地球的阳极催化剂。在这里,我们报告了对用于AEM装置的第一行过渡金属(羟基)氢氧化物/氧化物催化剂粉末的研究,并将物理性能和性能与基准IrO x催化剂以及碱性电解槽的典型催化剂进行了比较。我们表明,使用典型的三电极电池在碱性电解质中测得的催化剂的氧释放活性是AEM系统中性能的较差指标。NiFeO x是碱性电解液中最好的氧气释放反应(OER)催化剂ħ ÿ羟基氧化物,是其中固体碱性电解液用纯水进料一起使用在AEM电解装置最差。基于NiCoO x的催化剂在AEM电解中显示出最佳性能。通过在颗粒表面添加铁,可以进一步提高性能。我们将性能的差异部分归因于催化剂相的电导率差异,这也已进行了测量和报告。
更新日期:2018-11-15
中文翻译:

碱性阴离子交换膜水电解中的大地富氧电催化剂:催化剂电导率的影响以及三电极电池性能的比较
阴离子交换膜(AEM)电解是一种有希望的技术,它可以通过分解纯水来生产氢气。与质子交换膜(PEM)技术需要贵金属氧化物阳极相反,AEM系统允许使用富含地球的阳极催化剂。在这里,我们报告了对用于AEM装置的第一行过渡金属(羟基)氢氧化物/氧化物催化剂粉末的研究,并将物理性能和性能与基准IrO x催化剂以及碱性电解槽的典型催化剂进行了比较。我们表明,使用典型的三电极电池在碱性电解质中测得的催化剂的氧释放活性是AEM系统中性能的较差指标。NiFeO x是碱性电解液中最好的氧气释放反应(OER)催化剂ħ ÿ羟基氧化物,是其中固体碱性电解液用纯水进料一起使用在AEM电解装置最差。基于NiCoO x的催化剂在AEM电解中显示出最佳性能。通过在颗粒表面添加铁,可以进一步提高性能。我们将性能的差异部分归因于催化剂相的电导率差异,这也已进行了测量和报告。































 京公网安备 11010802027423号
京公网安备 11010802027423号