当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Clinical Candidate 2-(4-(2-((1H-Benzo[d]imidazol-2-yl)thio)ethyl)piperazin-1-yl)-N-(6-methyl-2,4-bis(methylthio)pyridin-3-yl)acetamide Hydrochloride [K-604], an Aqueous-Soluble Acyl-CoA:Cholesterol O-Acyltransferase-1 Inhibitor
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-11-15 00:00:00 , DOI: 10.1021/acs.jmedchem.8b01256 Kimiyuki Shibuya 1 , Katsumi Kawamine 1 , Chiyoka Ozaki 1 , Tadaaki Ohgiya 1 , Toshiyuki Edano 1 , Yasunobu Yoshinaka 1 , Yoshihiko Tsunenari 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-11-15 00:00:00 , DOI: 10.1021/acs.jmedchem.8b01256 Kimiyuki Shibuya 1 , Katsumi Kawamine 1 , Chiyoka Ozaki 1 , Tadaaki Ohgiya 1 , Toshiyuki Edano 1 , Yasunobu Yoshinaka 1 , Yoshihiko Tsunenari 1
Affiliation
|
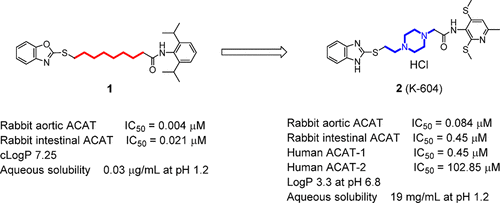
2-(4-(2-((1H-Benzo[d]imidazol-2-yl)thio)ethyl)piperazin-1-yl)-N-(6-methyl-2,4-bis(methylthio)pyridin-3-yl)acetamide hydrochloride (K-604, 2) has been identified as an aqueous-soluble potent inhibitor of human acyl-coenzyme A:cholesterol O-acyltransferase (ACAT, also known as SOAT)-1 that exhibits 229-fold selectivity for human ACAT-1 over human ACAT-2. In our molecular design, the insertion of a piperazine unit in place of a 6-methylene chain in the linker between the head (pyridylacetamide) and tail (benzimidazole) moieties led to a marked enhancement of the aqueous solubility (up to 19 mg/mL at pH 1.2) and a significant improvement of the oral absorption (the Cmax of 2 was 1100-fold higher than that of 1 in fasted dogs) compared with those of the previously selected compound, 1. After ensuring the pharmacological effects and safety, we designated 2 as a clinical candidate, named K-604. Considering the therapeutic results of ACAT inhibitors in past clinical trials, we believe that K-604 will be useful for the treatment of incurable diseases involving ACAT-1 overexpression.
中文翻译:

临床候选2-(4-(2-((1 H-苯并[ d ]咪唑-2-基)硫代)乙基)哌嗪-1-基)-N-(6-甲基-2,4-bis (甲硫基)吡啶-3-基)乙酰胺盐酸盐[K-604],水溶性酰基辅酶A:胆固醇O-酰基转移酶-1抑制剂
2-(4-(2-(((1 H-苯并[ d ]咪唑-2-基]硫代]乙基]哌嗪-1-基] -N-(6-甲基-2,4-双(甲硫基)]吡啶-3-基)乙酰胺盐酸盐(K-604,2)已被确定为人类酰基辅酶A的水性可溶强效抑制剂:胆固醇ö -acyltransferase(ACAT,也称为这项方案)-1表现出229倍对人ACAT-1的选择性高于对人ACAT-2的选择性。在我们的分子设计中,在头部(吡啶基乙酰胺)和尾部(苯并咪唑)之间的连接基中插入哌嗪单元代替6-亚甲基链导致水溶解度显着提高(最高19 mg / mL) pH 1.2时)和口服吸收的显着改善(C max为2为1100倍比的更高1与先前选择的化合物,相比于禁食的狗)1。在确保药理作用和安全性之后,我们指定2个临床候选药物,命名为K-604。考虑到ACAT抑制剂在过去的临床试验中的治疗结果,我们认为K-604将可用于治疗涉及ACAT-1过表达的不治之症。
更新日期:2018-11-15
中文翻译:

临床候选2-(4-(2-((1 H-苯并[ d ]咪唑-2-基)硫代)乙基)哌嗪-1-基)-N-(6-甲基-2,4-bis (甲硫基)吡啶-3-基)乙酰胺盐酸盐[K-604],水溶性酰基辅酶A:胆固醇O-酰基转移酶-1抑制剂
2-(4-(2-(((1 H-苯并[ d ]咪唑-2-基]硫代]乙基]哌嗪-1-基] -N-(6-甲基-2,4-双(甲硫基)]吡啶-3-基)乙酰胺盐酸盐(K-604,2)已被确定为人类酰基辅酶A的水性可溶强效抑制剂:胆固醇ö -acyltransferase(ACAT,也称为这项方案)-1表现出229倍对人ACAT-1的选择性高于对人ACAT-2的选择性。在我们的分子设计中,在头部(吡啶基乙酰胺)和尾部(苯并咪唑)之间的连接基中插入哌嗪单元代替6-亚甲基链导致水溶解度显着提高(最高19 mg / mL) pH 1.2时)和口服吸收的显着改善(C max为2为1100倍比的更高1与先前选择的化合物,相比于禁食的狗)1。在确保药理作用和安全性之后,我们指定2个临床候选药物,命名为K-604。考虑到ACAT抑制剂在过去的临床试验中的治疗结果,我们认为K-604将可用于治疗涉及ACAT-1过表达的不治之症。






























 京公网安备 11010802027423号
京公网安备 11010802027423号