当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photocatalytic Reduction of Carbon Dioxide to CO and HCO2H Using fac-Mn(CN)(bpy)(CO)3
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2016-03-01 00:00:00 , DOI: 10.1021/acs.inorgchem.6b00379
Po Ling Cheung 1 , Charles W. Machan 1 , Aramice Y. S. Malkhasian 2 , Jay Agarwal 3 , Clifford P. Kubiak 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2016-03-01 00:00:00 , DOI: 10.1021/acs.inorgchem.6b00379
Po Ling Cheung 1 , Charles W. Machan 1 , Aramice Y. S. Malkhasian 2 , Jay Agarwal 3 , Clifford P. Kubiak 1
Affiliation
|
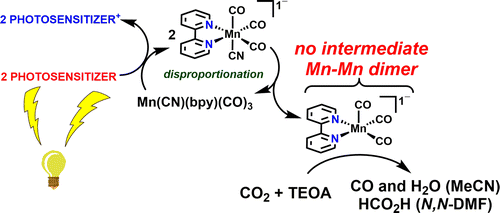
Studies are reported regarding the use of Mn(CN)(bpy)(CO)3 (1) as a catalyst for CO2 reduction employing [Ru(dmb)3]2+ as a photosensitizer in mixtures of dry N,N-dimethylformamide-triethanolamine (N,N-DMF-TEOA) or acetonitrile-TEOA (MeCN-TEOA) with 1-benzyl-1,4-dihydronicotinamide as a sacrificial reductant. Irradiation with 470 nm light for up to 15 h yields both CO and HCO2H with maximum turnover numbers (TONs) as high as 21 and 127, respectively, with product preference dependent on the solvent. Further data suggests that upon single electron reduction this catalyst avoids the formation of a Mn–Mn dimer and instead undergoes a disproportionation reaction, which requires 2 equiv of [Mn(CN)(bpy)(CO)3]•– to generate 1 equiv each of the active catalyst [Mn(bpy)(CO)3]− and the starting compound 1. Additional characterization by cyclic voltammetry (CV) and infrared spectroelectrochemistry (IR-SEC) indicates that the stability of the singly reduced [Mn(CN)(bpy)(CO)3]•– differs slightly in the N,N-DMF-TEOA solvent system compared to the MeCN-TEOA system. This contributes to the observed selectivities for HCO2H vs CO production.
中文翻译:

使用fac -Mn(CN)(bpy)(CO)3将二氧化碳光催化还原为CO和HCO 2 H
关于在干燥的N,N-二甲基甲酰胺的混合物中使用[Ru(dmb)3 ] 2+作为光敏剂,使用Mn(CN)(bpy)(CO)3(1)作为还原CO 2的催化剂的研究已有报道。-三乙醇胺(N,N -DMF-TEOA)或乙腈-TEOA(MeCN-TEOA),以1-苄基-1,4-二氢烟酰胺为牺牲还原剂。用470 nm的光照射长达15小时,会同时产生CO和HCO 2H的最大周转数(TONs)分别高达21和127,产品偏好取决于溶剂。进一步的数据表明,在单电子还原反应中,该催化剂避免了Mn-Mn二聚体的形成,而是发生歧化反应,这需要2当量的[Mn(CN)(bpy)(CO)3 ] •-生成1当量活性催化剂[Mn(bpy)(CO)3 ] -和起始化合物1中的每一个。通过循环伏安法(CV)和红外光谱电化学(IR-SEC)进行的其他表征表明,单还原的[Mn(CN)(bpy)(CO)3 ] •–的稳定性在N,N上略有不同-DMF-TEOA溶剂系统与MeCN-TEOA系统相比。这有助于观察到的HCO 2 H与CO生成的选择性。
更新日期:2016-03-01
中文翻译:

使用fac -Mn(CN)(bpy)(CO)3将二氧化碳光催化还原为CO和HCO 2 H
关于在干燥的N,N-二甲基甲酰胺的混合物中使用[Ru(dmb)3 ] 2+作为光敏剂,使用Mn(CN)(bpy)(CO)3(1)作为还原CO 2的催化剂的研究已有报道。-三乙醇胺(N,N -DMF-TEOA)或乙腈-TEOA(MeCN-TEOA),以1-苄基-1,4-二氢烟酰胺为牺牲还原剂。用470 nm的光照射长达15小时,会同时产生CO和HCO 2H的最大周转数(TONs)分别高达21和127,产品偏好取决于溶剂。进一步的数据表明,在单电子还原反应中,该催化剂避免了Mn-Mn二聚体的形成,而是发生歧化反应,这需要2当量的[Mn(CN)(bpy)(CO)3 ] •-生成1当量活性催化剂[Mn(bpy)(CO)3 ] -和起始化合物1中的每一个。通过循环伏安法(CV)和红外光谱电化学(IR-SEC)进行的其他表征表明,单还原的[Mn(CN)(bpy)(CO)3 ] •–的稳定性在N,N上略有不同-DMF-TEOA溶剂系统与MeCN-TEOA系统相比。这有助于观察到的HCO 2 H与CO生成的选择性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号