当前位置:
X-MOL 学术
›
Cancer Gene Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bacteria-mediated delivery of RNAi effector molecules against viral HPV16-E7 eradicates oral squamous carcinoma cells (OSCC) via apoptosis.
Cancer Gene Therapy ( IF 4.8 ) Pub Date : 2018-11-15 , DOI: 10.1038/s41417-018-0054-x Omar Bauomy Ahmed 1 , Hermann Lage 1, 2
Cancer Gene Therapy ( IF 4.8 ) Pub Date : 2018-11-15 , DOI: 10.1038/s41417-018-0054-x Omar Bauomy Ahmed 1 , Hermann Lage 1, 2
Affiliation
|
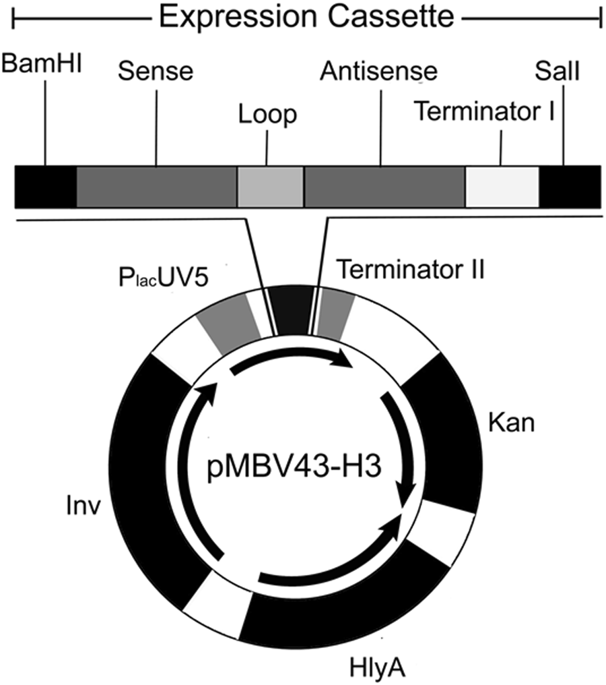
Delivery of RNAi-mediating shRNA molecules for gene silencing via bacteria, i.e. by transkingdom RNAi (tkRNAi) technology, is suggested to be a powerful alternative technique. In this work, the efficiency of bacterial delivery of shRNAs directed against HPV16-E7-specific mRNA to oral squamous carcinoma cells (OSCCs) was evaluated. E. coli were transfected with a plasmid encoding the inv locus and the Hlya gene to enable the bacteria to enter carcinoma cells and to escape from endocytotic vesicles. The bacterial penetration to the target cells was confirmed by DAPI staining. The HPV16-E7 mRNA expression in bacteria-treated OSCCs dropped to 61% of the controls as measured by qRT-PCR. Corresponding inhibition of the HPV16-E7 protein was confirmed by western blotting. The IC50 of bacteria-treated OSCCs was reduced to more than 75%. Flow cytometry assays showed higher total apoptosis and caspase-3 activation (6.6-fold and 8.4-fold respectively) in OSCCs following exposure to anti-HPV-E7 bacteria compared to anti-GFP bacteria (2-fold and 2.9-fold, respectively). In conclusion, it was demonstrated for the first time that tkRNAi technology is also useful for treatment of squamous carcinoma cells. Anti-HPV16-E7 shRNA-encoding bacteria can efficiently deliver RNAi effectors to OSCCs mediating a strong and specific gene silencing associated with triggering cell death.
中文翻译:

细菌介导的针对病毒HPV16-E7的RNAi效应分子的传递通过凋亡消除了口腔鳞状细胞癌细胞(OSCC)。
RNAi介导的shRNA分子通过细菌的传递,即通过transkingdom RNAi(tkRNAi)技术进行基因沉默,被认为是一种强大的替代技术。在这项工作中,评估了将针对HPV16-E7特异性mRNA的shRNA细菌递送至口腔鳞状细胞癌(OSCC)的效率。用编码inv基因座和Hlya基因的质粒转染大肠杆菌,以使细菌能够进入癌细胞并从胞吞小泡中逃逸。通过DAPI染色确认细菌渗透至靶细胞。经qRT-PCR测定,细菌处理的OSCC中的HPV16-E7 mRNA表达下降至对照的61%。通过蛋白质印迹证实了对HPV16-E7蛋白的相应抑制。细菌处理过的OSCC的IC50降低到75%以上。流式细胞仪分析显示,与抗GFP细菌相比,暴露于抗HPV-E7细菌后OSCC中的总细胞凋亡和caspase-3活化更高(分别为2倍和2.9倍) 。总之,首次证明tkRNAi技术也可用于治疗鳞状癌细胞。编码抗HPV16-E7 shRNA的细菌可以有效地将RNAi效应子传递至OSCC,从而介导与触发细胞死亡相关的强烈而特异性的基因沉默。首次证明,tkRNAi技术也可用于治疗鳞状癌细胞。编码抗HPV16-E7 shRNA的细菌可以有效地将RNAi效应子传递至OSCC,从而介导与触发细胞死亡相关的强烈而特异性的基因沉默。首次证明,tkRNAi技术也可用于治疗鳞状癌细胞。编码抗HPV16-E7 shRNA的细菌可以有效地将RNAi效应子传递至OSCC,从而介导与触发细胞死亡相关的强烈而特异性的基因沉默。
更新日期:2019-05-16
中文翻译:

细菌介导的针对病毒HPV16-E7的RNAi效应分子的传递通过凋亡消除了口腔鳞状细胞癌细胞(OSCC)。
RNAi介导的shRNA分子通过细菌的传递,即通过transkingdom RNAi(tkRNAi)技术进行基因沉默,被认为是一种强大的替代技术。在这项工作中,评估了将针对HPV16-E7特异性mRNA的shRNA细菌递送至口腔鳞状细胞癌(OSCC)的效率。用编码inv基因座和Hlya基因的质粒转染大肠杆菌,以使细菌能够进入癌细胞并从胞吞小泡中逃逸。通过DAPI染色确认细菌渗透至靶细胞。经qRT-PCR测定,细菌处理的OSCC中的HPV16-E7 mRNA表达下降至对照的61%。通过蛋白质印迹证实了对HPV16-E7蛋白的相应抑制。细菌处理过的OSCC的IC50降低到75%以上。流式细胞仪分析显示,与抗GFP细菌相比,暴露于抗HPV-E7细菌后OSCC中的总细胞凋亡和caspase-3活化更高(分别为2倍和2.9倍) 。总之,首次证明tkRNAi技术也可用于治疗鳞状癌细胞。编码抗HPV16-E7 shRNA的细菌可以有效地将RNAi效应子传递至OSCC,从而介导与触发细胞死亡相关的强烈而特异性的基因沉默。首次证明,tkRNAi技术也可用于治疗鳞状癌细胞。编码抗HPV16-E7 shRNA的细菌可以有效地将RNAi效应子传递至OSCC,从而介导与触发细胞死亡相关的强烈而特异性的基因沉默。首次证明,tkRNAi技术也可用于治疗鳞状癌细胞。编码抗HPV16-E7 shRNA的细菌可以有效地将RNAi效应子传递至OSCC,从而介导与触发细胞死亡相关的强烈而特异性的基因沉默。































 京公网安备 11010802027423号
京公网安备 11010802027423号