当前位置:
X-MOL 学术
›
Acta Pharmacol. Sin.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Different structures of berberine and five other protoberberine alkaloids that affect P-glycoprotein-mediated efflux capacity.
Acta Pharmacologica Sinica ( IF 6.9 ) Pub Date : 2018-11-15 , DOI: 10.1038/s41401-018-0183-7
Yi-Ting Zhang 1, 2 , Yu-Qi Yu 1, 2 , Xiao-Xia Yan 1, 2 , Wen-Jie Wang 1, 2 , Xiao-Ting Tian 1 , Le Wang 1, 2 , Wei-Liang Zhu 1, 2 , Li-Kun Gong 1, 2 , Guo-Yu Pan 1, 2
Acta Pharmacologica Sinica ( IF 6.9 ) Pub Date : 2018-11-15 , DOI: 10.1038/s41401-018-0183-7
Yi-Ting Zhang 1, 2 , Yu-Qi Yu 1, 2 , Xiao-Xia Yan 1, 2 , Wen-Jie Wang 1, 2 , Xiao-Ting Tian 1 , Le Wang 1, 2 , Wei-Liang Zhu 1, 2 , Li-Kun Gong 1, 2 , Guo-Yu Pan 1, 2
Affiliation
|
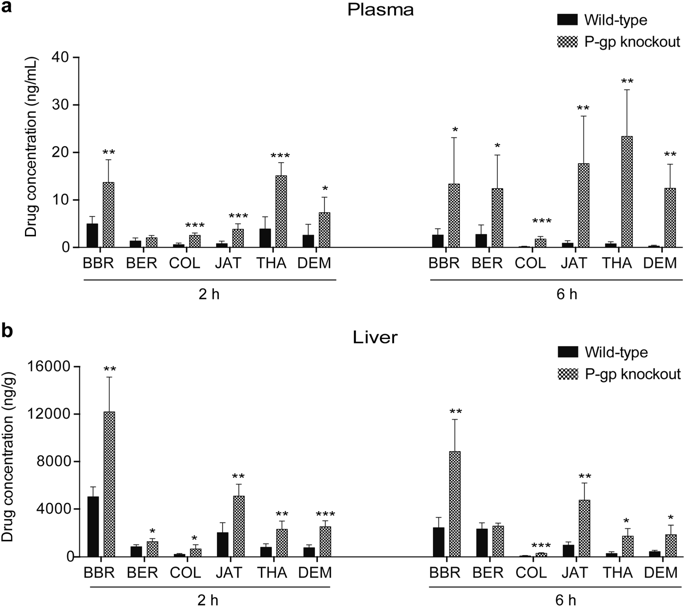
Berberine, berberrubine, thalifendine, demethyleneberberine, jatrorrhizine, and columbamine are six natural protoberberine alkaloid (PA) compounds that display extensive pharmacological properties and share the same protoberberine molecular skeleton with only slight substitution differences. The oral delivery of most PAs is hindered by their poor bioavailability, which is largely caused by P-glycoprotein (P-gp)-mediated drug efflux. Meanwhile, P-gp undergoes large-scale conformational changes (from an inward-facing to an outward-facing state) when transporting substrates, and these changes might strongly affect the P-gp-binding specificity. To confirm whether these six compounds are substrates of P-gp, to investigate the differences in efflux capacity caused by their trivial structural differences and to reveal the key to increasing their binding affinity to P-gp, we conducted a series of in vivo, in vitro, and in silico assays. Here, we first confirmed that all six compounds were substrates of P-gp by comparing the drug concentrations in wild-type and P-gp-knockout mice in vivo. The efflux capacity (net efflux) ranked as berberrubine > berberine > columbamine ~ jatrorrhizine > thalifendine > demethyleneberberine based on in vitro transport studies in Caco-2 monolayers. Using molecular dynamics simulation and molecular docking techniques, we determined the transport pathways of the six compounds and their binding affinities to P-gp. The results suggested that at the early binding stage, different hydrophobic and electrostatic interactions collectively differentiate the binding affinities of the compounds to P-gp, whereas electrostatic interactions are the main determinant at the late release stage. In addition to hydrophobic interactions, hydrogen bonds play an important role in discriminating the binding affinities.
中文翻译:

小structures碱和五个其他原小ber碱生物碱的不同结构,会影响P-糖蛋白介导的外排能力。
小ber碱,小ber红素,thalifendine,脱甲基小ber碱,麻风树碱和哥伦巴胺是六种天然的小to碱生物碱(PA)化合物,它们具有广泛的药理特性,并且具有相同的小ber碱分子骨架,但仅有很小的取代差异。大多数PA的口服传递受到其生物利用度差的阻碍,这主要是由P-糖蛋白(P-gp)介导的药物流出引起的。同时,在运输底物时,P-gp发生大规模的构象变化(从向内到向外的状态),这些变化可能强烈影响P-gp的结合特异性。为了确认这六种化合物是否为P-gp的底物,为了研究由它们琐碎的结构差异引起的外排容量差异,并揭示增加它们与P-gp结合亲和力的关键,我们进行了一系列体内,体外和计算机分析。在这里,我们首先通过比较野生型和P-gp基因敲除小鼠体内的药物浓度,来确定所有6种化合物都是P-gp的底物。根据在Caco-2单层中进行的体外转运研究,流出容量(净流出)依次为小>红素>小ber碱>哥伦巴胺〜麻风树碱> thalifendine>脱亚甲基小ber碱。使用分子动力学模拟和分子对接技术,我们确定了这六种化合物的转运途径及其与P-gp的结合亲和力。结果表明,在绑定的早期,不同的疏水和静电相互作用共同区分了化合物与P-gp的结合亲和力,而静电相互作用是释放后期的主要决定因素。除疏水相互作用外,氢键在区分结合亲和力中也起重要作用。
更新日期:2019-01-26
中文翻译:

小structures碱和五个其他原小ber碱生物碱的不同结构,会影响P-糖蛋白介导的外排能力。
小ber碱,小ber红素,thalifendine,脱甲基小ber碱,麻风树碱和哥伦巴胺是六种天然的小to碱生物碱(PA)化合物,它们具有广泛的药理特性,并且具有相同的小ber碱分子骨架,但仅有很小的取代差异。大多数PA的口服传递受到其生物利用度差的阻碍,这主要是由P-糖蛋白(P-gp)介导的药物流出引起的。同时,在运输底物时,P-gp发生大规模的构象变化(从向内到向外的状态),这些变化可能强烈影响P-gp的结合特异性。为了确认这六种化合物是否为P-gp的底物,为了研究由它们琐碎的结构差异引起的外排容量差异,并揭示增加它们与P-gp结合亲和力的关键,我们进行了一系列体内,体外和计算机分析。在这里,我们首先通过比较野生型和P-gp基因敲除小鼠体内的药物浓度,来确定所有6种化合物都是P-gp的底物。根据在Caco-2单层中进行的体外转运研究,流出容量(净流出)依次为小>红素>小ber碱>哥伦巴胺〜麻风树碱> thalifendine>脱亚甲基小ber碱。使用分子动力学模拟和分子对接技术,我们确定了这六种化合物的转运途径及其与P-gp的结合亲和力。结果表明,在绑定的早期,不同的疏水和静电相互作用共同区分了化合物与P-gp的结合亲和力,而静电相互作用是释放后期的主要决定因素。除疏水相互作用外,氢键在区分结合亲和力中也起重要作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号