当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electronic Structure Modulation of Graphitic Carbon Nitride by Oxygen Doping for Enhanced Catalytic Degradation of Organic Pollutants through Peroxymonosulfate Activation
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-12-03 , DOI: 10.1021/acs.est.8b05246 Yaowen Gao 1 , Yue Zhu 1 , Lai Lyu 1 , Qingyi Zeng 1 , Xueci Xing 1 , Chun Hu 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-12-03 , DOI: 10.1021/acs.est.8b05246 Yaowen Gao 1 , Yue Zhu 1 , Lai Lyu 1 , Qingyi Zeng 1 , Xueci Xing 1 , Chun Hu 1
Affiliation
|
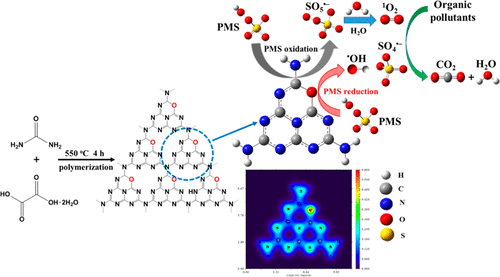
Oxygen-doped graphitic carbon nitride (O–CN) was fabricated via a facile thermal polymerization method using urea and oxalic acid dihydrate as the graphitic carbon nitride precursor and oxygen source, respectively. Experimental and theoretical results revealed that oxygen doping preferentially occurred on the two-coordinated nitrogen positions, which create the formation of low and high electron density areas resulting in the electronic structure modulation of O–CN. As a result, the resultant O–CN exhibits enhanced catalytic activity and excellent long-term stability for peroxymonosulfate (PMS) activation toward the degradation of organic pollutants. The O–CN with modulated electronic structure enables PMS oxidation over the electron-deficient C atoms for the generation of singlet oxygen (1O2) and PMS reduction around the electron-rich O dopants for the formation of hydroxyl radical (•OH) and sulfate radical (SO4•–), in which 1O2 is the major reactive oxygen species, contributing to the selective reactivity of the O–CN/PMS system. Our findings not only propose a novel PMS activation mechanism in terms of simultaneous PMS oxidation and reduction for the production of nonradical and radical species but also provide a valuable insight for the development of efficient metal-free catalysts through nonmetal doping toward the persulfate-based environmental cleanup.
中文翻译:

氧掺杂对石墨化氮化碳的电子结构调节,以通过过氧一硫酸盐活化促进有机污染物的催化降解
通过尿素和草酸二水合物分别作为石墨氮化碳前驱体和氧源,通过简便的热聚合方法制备了氧掺杂石墨氮化碳(O-CN)。实验和理论结果表明,氧掺杂优先发生在氮的两个配位位置上,从而形成低和高电子密度区域,从而导致O-CN的电子结构发生调制。结果,所得的O-CN表现出增强的催化活性,并具有对过氧单硫酸盐(PMS)活化以降解有机污染物的优异长期稳定性。具有调制电子结构的O–CN可使PMS在缺电子的C原子上氧化,从而生成单线态氧(1 O 2)和PMS在富电子的O掺杂剂周围还原,从而形成羟基自由基(• OH)和硫酸盐自由基(SO 4 •–),其中1 O 2是主要的活性氧种类,这有助于氧的选择性反应。 O–CN / PMS系统。我们的发现不仅提出了一种同时发生PMS氧化和还原以生产非自由基和自由基的新型PMS活化机理,而且还为通过向过硫酸盐基环境中进行非金属掺杂而开发有效的无金属催化剂提供了宝贵的见解。清理。
更新日期:2018-12-03
中文翻译:

氧掺杂对石墨化氮化碳的电子结构调节,以通过过氧一硫酸盐活化促进有机污染物的催化降解
通过尿素和草酸二水合物分别作为石墨氮化碳前驱体和氧源,通过简便的热聚合方法制备了氧掺杂石墨氮化碳(O-CN)。实验和理论结果表明,氧掺杂优先发生在氮的两个配位位置上,从而形成低和高电子密度区域,从而导致O-CN的电子结构发生调制。结果,所得的O-CN表现出增强的催化活性,并具有对过氧单硫酸盐(PMS)活化以降解有机污染物的优异长期稳定性。具有调制电子结构的O–CN可使PMS在缺电子的C原子上氧化,从而生成单线态氧(1 O 2)和PMS在富电子的O掺杂剂周围还原,从而形成羟基自由基(• OH)和硫酸盐自由基(SO 4 •–),其中1 O 2是主要的活性氧种类,这有助于氧的选择性反应。 O–CN / PMS系统。我们的发现不仅提出了一种同时发生PMS氧化和还原以生产非自由基和自由基的新型PMS活化机理,而且还为通过向过硫酸盐基环境中进行非金属掺杂而开发有效的无金属催化剂提供了宝贵的见解。清理。

































 京公网安备 11010802027423号
京公网安备 11010802027423号