Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Blocking nuclear export of HSPA8 after heat shock stress severely alters cell survival.
Scientific Reports ( IF 3.8 ) Pub Date : 2018-Nov-14 , DOI: 10.1038/s41598-018-34887-6 Fengjuan Wang , Srinivasa Reddy Bonam , Nicolas Schall , Lauriane Kuhn , Philippe Hammann , Olivier Chaloin , Jean-Baptiste Madinier , Jean-Paul Briand , Nicolas Page , Sylviane Muller
Scientific Reports ( IF 3.8 ) Pub Date : 2018-Nov-14 , DOI: 10.1038/s41598-018-34887-6 Fengjuan Wang , Srinivasa Reddy Bonam , Nicolas Schall , Lauriane Kuhn , Philippe Hammann , Olivier Chaloin , Jean-Baptiste Madinier , Jean-Paul Briand , Nicolas Page , Sylviane Muller
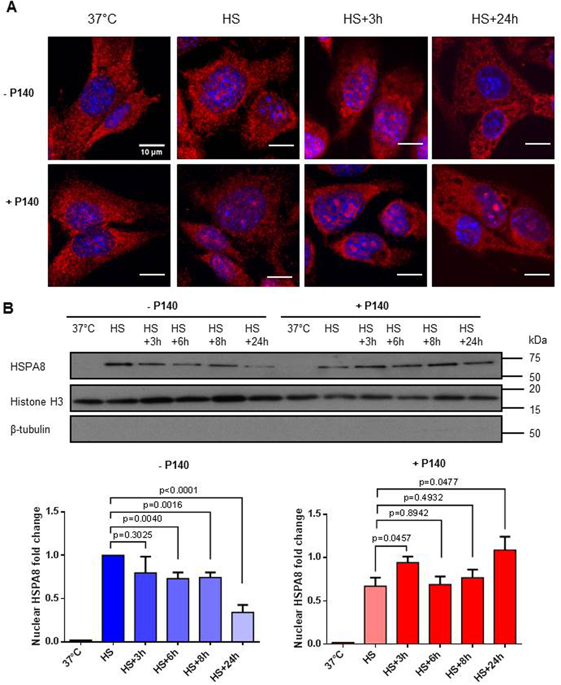
|
The nuclear translocation of endogenous heat shock cognate protein HSPA8 is a requisite for cell survival during oxidative and heat shock stress. Upon these events, cytoplasmic HSPA8 is thought to concentrate within the nucleus and nucleolus. When the situation returns to normal, HSPA8 is released from its nuclear/nucleolar anchors and redistributes into the cytoplasm. By using different stress conditions and a 21-mer phosphopeptide tool called P140, which binds HSPA8 and hampers its chaperone properties, we deciphered the cellular and molecular effects arising during this vital cytoplasmic-nuclear-cytoplasmic shuttling process. Using the non-metastatic fibroblastoid cell line MRL/N-1 derived from a MRL/MpTn-gld/gld lupus-prone mouse, we discovered that P140 treatment neutralized the egress of HSPA8 from nucleus to cytoplasm in the cell recovery phase. This lack of relocation of HSPA8 into the cytoplasm of heat-shocked MRL/N-1 cells altered the ability of these cells to survive when a second mild oxidative stress mimicking inflammatory conditions was applied. Crosslinking experiments followed by proteomics studies showed that P140 binds regions close to nuclear import and export signal sequences encompassed within the HSPA8 structure. These data are consistent with HSPA8 having a crucial cell protective role against reactive oxygen species (ROS) production by mitochondria during inflammatory conditions.
中文翻译:

热休克应激后阻止HSPA8的核输出会严重改变细胞存活率。
内源性热休克同源蛋白HSPA8的核易位是氧化和热休克应激期间细胞存活的必要条件。一旦发生这些事件,就认为细胞质HSPA8集中在细胞核和核仁中。当情况恢复正常时,HSPA8从其核/核仁锚释放,并重新分布到细胞质中。通过使用不同的应激条件和称为P140的21-聚磷酸肽工具,该工具结合HSPA8并阻碍其分子伴侣性质,我们破译了在此重要的胞质-核-胞质穿梭过程中产生的细胞和分子效应。使用源自MRL / MpTn-gld / gld狼疮易感小鼠的非转移性成纤维细胞系MRL / N-1,我们发现P140处理在细胞恢复阶段中和了HSPA8从细胞核到细胞质的释放。HSPA8不能重定位到热激MRL / N-1细胞的细胞质中,从而改变了这些细胞在模拟炎症条件的第二次轻度氧化应激下存活的能力。蛋白质组学研究之后的交联实验表明,P140结合了HSPA8结构中所包含的靠近核进出口信号序列的区域。这些数据与HSPA8在炎症条件下对线粒体产生的活性氧(ROS)具有至关重要的细胞保护作用相一致。HSPA8不能重定位到热激MRL / N-1细胞的细胞质中,从而改变了这些细胞在模拟炎症条件的第二次轻度氧化应激下存活的能力。蛋白质组学研究之后的交联实验表明,P140结合了HSPA8结构中所包含的靠近核进出口信号序列的区域。这些数据与HSPA8在炎症条件下对线粒体产生的活性氧(ROS)具有至关重要的细胞保护作用相一致。HSPA8不能重定位到热激MRL / N-1细胞的细胞质中,从而改变了这些细胞在模拟炎症条件的第二次轻度氧化应激下存活的能力。蛋白质组学研究之后的交联实验表明,P140结合了HSPA8结构中所包含的靠近核输入和输出信号序列的区域。这些数据与HSPA8在炎症条件下对线粒体产生的活性氧(ROS)具有至关重要的细胞保护作用相一致。
更新日期:2018-11-14
中文翻译:

热休克应激后阻止HSPA8的核输出会严重改变细胞存活率。
内源性热休克同源蛋白HSPA8的核易位是氧化和热休克应激期间细胞存活的必要条件。一旦发生这些事件,就认为细胞质HSPA8集中在细胞核和核仁中。当情况恢复正常时,HSPA8从其核/核仁锚释放,并重新分布到细胞质中。通过使用不同的应激条件和称为P140的21-聚磷酸肽工具,该工具结合HSPA8并阻碍其分子伴侣性质,我们破译了在此重要的胞质-核-胞质穿梭过程中产生的细胞和分子效应。使用源自MRL / MpTn-gld / gld狼疮易感小鼠的非转移性成纤维细胞系MRL / N-1,我们发现P140处理在细胞恢复阶段中和了HSPA8从细胞核到细胞质的释放。HSPA8不能重定位到热激MRL / N-1细胞的细胞质中,从而改变了这些细胞在模拟炎症条件的第二次轻度氧化应激下存活的能力。蛋白质组学研究之后的交联实验表明,P140结合了HSPA8结构中所包含的靠近核进出口信号序列的区域。这些数据与HSPA8在炎症条件下对线粒体产生的活性氧(ROS)具有至关重要的细胞保护作用相一致。HSPA8不能重定位到热激MRL / N-1细胞的细胞质中,从而改变了这些细胞在模拟炎症条件的第二次轻度氧化应激下存活的能力。蛋白质组学研究之后的交联实验表明,P140结合了HSPA8结构中所包含的靠近核进出口信号序列的区域。这些数据与HSPA8在炎症条件下对线粒体产生的活性氧(ROS)具有至关重要的细胞保护作用相一致。HSPA8不能重定位到热激MRL / N-1细胞的细胞质中,从而改变了这些细胞在模拟炎症条件的第二次轻度氧化应激下存活的能力。蛋白质组学研究之后的交联实验表明,P140结合了HSPA8结构中所包含的靠近核输入和输出信号序列的区域。这些数据与HSPA8在炎症条件下对线粒体产生的活性氧(ROS)具有至关重要的细胞保护作用相一致。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号