当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Making the SF5 Group More Accessible: A Gas‐Reagent‐Free Approach to Aryl Tetrafluoro‐λ6‐sulfanyl Chlorides
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-01-18 , DOI: 10.1002/anie.201812356
Cody Ross Pitts 1 , Dustin Bornemann 1 , Phil Liebing 1 , Nico Santschi 1 , Antonio Togni 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-01-18 , DOI: 10.1002/anie.201812356
Cody Ross Pitts 1 , Dustin Bornemann 1 , Phil Liebing 1 , Nico Santschi 1 , Antonio Togni 1
Affiliation
|
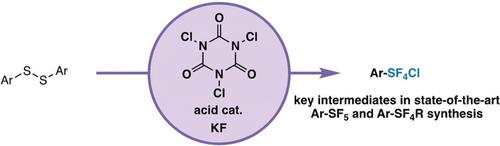
Modern pentafluorosulfanyl (SF5) chemistry has an Achilles heel: synthetic accessibility. Herein, we present the first approach to aryl‐SF4Cl compounds (key intermediates in state‐of‐the‐art aryl‐SF5 synthesis) that overcomes the reliance on hazardous fluorinating reagents and/or gas reagents (e.g. Cl2) by employing easy‐to‐handle trichloroisocyanuric acid, potassium fluoride, and catalytic amounts of acid. These simple, mild conditions allow direct access to aryl‐SF4Cl intermediates that either have not been or cannot be demonstrated using previous methods. Furthermore, the same approach provides access to aryl‐SF3 and aryl‐SeF3 compounds, which extend the applications of this chemistry beyond arene SF5‐functionalization, and demonstrate its ability to address a more general oxidative fluorination problem.
中文翻译:

使SF5组更易接近:芳烃四氟λ6-硫烷基氯的无气体试剂方法
现代五氟硫烷基(SF 5)化学的致命弱点是:合成材料的可及性。本文中,我们介绍了解决芳基-SF 4 Cl化合物(最新的芳基-SF 5合成中的关键中间体)的第一种方法,该方法克服了对有害氟化试剂和/或气体试剂(例如Cl 2)的依赖使用易于处理的三氯异氰尿酸,氟化钾和催化量的酸。这些简单,温和的条件使您可以直接接触到尚未使用或无法通过以前的方法证明的芳基-SF 4 Cl中间体。此外,相同的方法提供了访问芳-SF 3和芳基- SEF 3化合物,将这种化学的应用范围扩展到了芳烃SF 5官能化以外,并证明了其解决更普遍的氧化氟化问题的能力。
更新日期:2019-01-18
中文翻译:

使SF5组更易接近:芳烃四氟λ6-硫烷基氯的无气体试剂方法
现代五氟硫烷基(SF 5)化学的致命弱点是:合成材料的可及性。本文中,我们介绍了解决芳基-SF 4 Cl化合物(最新的芳基-SF 5合成中的关键中间体)的第一种方法,该方法克服了对有害氟化试剂和/或气体试剂(例如Cl 2)的依赖使用易于处理的三氯异氰尿酸,氟化钾和催化量的酸。这些简单,温和的条件使您可以直接接触到尚未使用或无法通过以前的方法证明的芳基-SF 4 Cl中间体。此外,相同的方法提供了访问芳-SF 3和芳基- SEF 3化合物,将这种化学的应用范围扩展到了芳烃SF 5官能化以外,并证明了其解决更普遍的氧化氟化问题的能力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号