当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Properties and Vibrational Spectra of Ethylammonium Nitrate Ionic Liquid Confined in Single-Walled Carbon Nanotubes
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-02-29 00:00:00 , DOI: 10.1021/acs.jpcc.6b00307
Guobing Zhou 1 , Yunzhi Li 1 , Zhen Yang 1 , Fangjia Fu 1 , Yiping Huang 1 , Zheng Wan 1 , Li Li 1 , Xiangshu Chen 1 , Na Hu 1 , Liangliang Huang 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-02-29 00:00:00 , DOI: 10.1021/acs.jpcc.6b00307
Guobing Zhou 1 , Yunzhi Li 1 , Zhen Yang 1 , Fangjia Fu 1 , Yiping Huang 1 , Zheng Wan 1 , Li Li 1 , Xiangshu Chen 1 , Na Hu 1 , Liangliang Huang 2
Affiliation
|
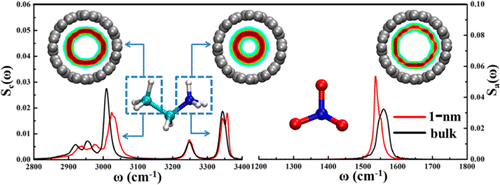
The structures and relevant vibrational spectra of an ethylammonium nitrate (EAN) ionic liquid (IL) confined in single-walled carbon nanotubes (SWCNTs) with various diameters have been investigated in detail by using classical molecular dynamics simulation. Our simulation results demonstrate that the EAN IL confined in larger SWCNTs can form well-defined multishell structures with an additional cation chain located at the center. However, a different single-shell hollow structure has been found for both the cations and the anions in the 1 nm SWCNT. For the cations confined in SWCNTs, the CH3 groups stay closer to the nanotube walls because of their solvophobic nature, while the NH3+ groups prefer to point toward the central axis. Accordingly, the NO3– anions tend to lean on the SWCNT surface with three O atoms facing the central axis to form hydrogen bonds (HBs) with the NH3+ groups. In addition, in the 1 nm SWCNT, the CH3 groups of cations exhibit an obvious blue shift of around 16 cm–1 for the C–H stretching mode with respect to the bulk value, and the N–H stretching mode of NH3+ groups is split into two characteristic peaks with one peak appearing at a higher frequency. Such a blue shift is attributed to the existence of more free space for the C–H bonds of confined CH3 groups, while the splitting phenomenon is due to the fact that more than 60% of the confined NH3+ groups have one dangling N–H bond. For the anions confined in the 1 nm SWCNT, the N–O stretching mode of NO3– has a maximum red shift of around 24 cm–1 with respect to the bulk value, which is attributed to enhanced HBs between anions and cations. Our simulation results reveal a molecular-level correlation between confined structural configurations and the corresponding vibrational spectra changes for the ILs confined in nanometer scale environments.
中文翻译:

单壁碳纳米管中的硝酸乙铵离子液体的结构性质和振动光谱
通过经典的分子动力学模拟,详细研究了局限在各种直径的单壁碳纳米管(SWCNT)中的硝酸乙基铵(EAN)离子液体(IL)的结构和相关的振动光谱。我们的模拟结果表明,局限在较大的SWCNT中的EAN IL可以形成轮廓分明的多壳结构,中心处有一条额外的阳离子链。但是,对于1 nm SWCNT中的阳离子和阴离子,已经发现了不同的单壳中空结构。对于SWCNT中的阳离子,由于其疏溶剂性,CH 3基团保持更靠近纳米管壁,而NH 3 +基团更倾向于指向中心轴。因此,NO 3–阴离子倾向于以三个O原子面向中心轴的方式靠在SWCNT表面上,从而与NH 3 +基团形成氢键(HBs)。此外,在1 nm SWCNT中,CH 3拉伸模式的CH 3阳离子相对于整体值和NH 3的NH拉伸模式表现出明显的蓝移,约为16 cm –1。+组被分为两个特征峰,其中一个峰出现在较高的频率。这种蓝移归因于受限的CH 3基团的C–H键存在更多的自由空间,而分裂现象是由于60%以上的受限NH 3 +各族之间有一个悬挂的NH键。对于在1个纳米SWCNT限定的阴离子,所述NO拉伸NO的模式3 -具有约24厘米的最大红移-1相对于所述本体的值,这归因于阴离子和阳离子之间的增强的HBs抗体。我们的模拟结果显示,在纳米级环境中,IL的局限性结构构型与相应的振动光谱变化之间存在分子水平的相关性。
更新日期:2016-02-29
中文翻译:

单壁碳纳米管中的硝酸乙铵离子液体的结构性质和振动光谱
通过经典的分子动力学模拟,详细研究了局限在各种直径的单壁碳纳米管(SWCNT)中的硝酸乙基铵(EAN)离子液体(IL)的结构和相关的振动光谱。我们的模拟结果表明,局限在较大的SWCNT中的EAN IL可以形成轮廓分明的多壳结构,中心处有一条额外的阳离子链。但是,对于1 nm SWCNT中的阳离子和阴离子,已经发现了不同的单壳中空结构。对于SWCNT中的阳离子,由于其疏溶剂性,CH 3基团保持更靠近纳米管壁,而NH 3 +基团更倾向于指向中心轴。因此,NO 3–阴离子倾向于以三个O原子面向中心轴的方式靠在SWCNT表面上,从而与NH 3 +基团形成氢键(HBs)。此外,在1 nm SWCNT中,CH 3拉伸模式的CH 3阳离子相对于整体值和NH 3的NH拉伸模式表现出明显的蓝移,约为16 cm –1。+组被分为两个特征峰,其中一个峰出现在较高的频率。这种蓝移归因于受限的CH 3基团的C–H键存在更多的自由空间,而分裂现象是由于60%以上的受限NH 3 +各族之间有一个悬挂的NH键。对于在1个纳米SWCNT限定的阴离子,所述NO拉伸NO的模式3 -具有约24厘米的最大红移-1相对于所述本体的值,这归因于阴离子和阳离子之间的增强的HBs抗体。我们的模拟结果显示,在纳米级环境中,IL的局限性结构构型与相应的振动光谱变化之间存在分子水平的相关性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号