当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Mechanism and Solvation Effect of Supramolecular Catalysis in a Synthetic Cavitand Receptor with an Inwardly Directed Carboxylic Acid for Ring-Opening Cyclization of Epoxy Alcohols
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-11-12 00:00:00 , DOI: 10.1021/acscatal.8b03256
Lina Xu 1 , Guoyong Fang 1, 2 , Junbin Tao 1 , Zihang Ye 1 , Sainan Xu 1 , Zhenyu Li 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-11-12 00:00:00 , DOI: 10.1021/acscatal.8b03256
Lina Xu 1 , Guoyong Fang 1, 2 , Junbin Tao 1 , Zihang Ye 1 , Sainan Xu 1 , Zhenyu Li 2
Affiliation
|
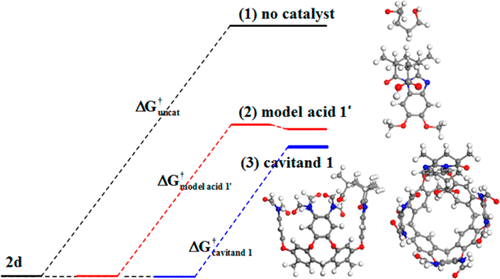
Supramolecular catalysis has become a hot topic in chemistry. To understand the origin of supramolecular catalysis, the catalytic mechanism of a synthetic cavitand receptor with an inwardly directed carboxylic acid for ring-opening cyclization of epoxy alcohols was investigated by performing density functional theory (DFT) calculations. The results reveal that the epoxy alcohol can be ring-opened via backside attack of the hydroxyl group to the epoxy group and cyclized in 5-exo and 6-endo modes. The carboxylic acid can catalyze the ring-opening cyclization of the epoxy alcohol through organocatalysis, since the carboxyl group can easily provide a proton to protonate the epoxy group. The synthetic cavitand receptor with an inwardly directed carboxylic acid can further catalyze the ring-opening cyclization of an epoxy alcohol through organocatalysis and supramolecular catalysis as a result of the hydrogen-bonding and C–H···π interactions formed between the receptor and substrate. Furthermore, the special structure and relative orientation of the substrate and the host can result in a good regioselectivity of supramolecular catalysis. In addition, the solvation analysis indicates that the solvation role has an effect on the supramolecular catalysis. The full solution-phase optimization of all species in the supramolecular catalytic reaction is given, and characterization of the solvation effect on the reaction barriers is also proposed. These insights into the catalytic mechanism and the solvation effect of the synthetic cavitand receptor may be applied to the design and synthesis of new and effective supramolecular catalysts.
中文翻译:

超分子催化合成的Cavitand受体与内向型羧酸的开环环化的分子机理和溶剂化作用。
超分子催化已经成为化学领域的热门话题。为了了解超分子催化的起源,通过执行密度泛函理论(DFT)计算,研究了具有向内定向羧酸的合成cavitand受体对环氧醇的开环环化的催化机理。结果表明,环氧醇可通过羟基对环氧环的背面攻击而开环,并在5- exo和6- endo环化模式。羧酸可通过有机催化来催化环氧醇的开环环化,因为羧基可容易地提供质子以使环氧基质子化。带有内向羧酸的合成腔体受体可以通过有机键合和超分子催化(由于氢键和C –在受体和底物之间形成了H···π相互作用。此外,底物与主体的特殊结构和相对取向可导致超分子催化的良好区域选择性。另外,溶剂化分析表明溶剂化作用对超分子催化有影响。给出了超分子催化反应中所有物质的全溶液相优化,并提出了对反应势垒的溶剂化作用的表征。这些对合成cavitand受体的催化机理和溶剂化作用的见解可用于设计和合成新型有效的超分子催化剂。
更新日期:2018-11-12
中文翻译:

超分子催化合成的Cavitand受体与内向型羧酸的开环环化的分子机理和溶剂化作用。
超分子催化已经成为化学领域的热门话题。为了了解超分子催化的起源,通过执行密度泛函理论(DFT)计算,研究了具有向内定向羧酸的合成cavitand受体对环氧醇的开环环化的催化机理。结果表明,环氧醇可通过羟基对环氧环的背面攻击而开环,并在5- exo和6- endo环化模式。羧酸可通过有机催化来催化环氧醇的开环环化,因为羧基可容易地提供质子以使环氧基质子化。带有内向羧酸的合成腔体受体可以通过有机键合和超分子催化(由于氢键和C –在受体和底物之间形成了H···π相互作用。此外,底物与主体的特殊结构和相对取向可导致超分子催化的良好区域选择性。另外,溶剂化分析表明溶剂化作用对超分子催化有影响。给出了超分子催化反应中所有物质的全溶液相优化,并提出了对反应势垒的溶剂化作用的表征。这些对合成cavitand受体的催化机理和溶剂化作用的见解可用于设计和合成新型有效的超分子催化剂。




































 京公网安备 11010802027423号
京公网安备 11010802027423号